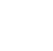6 Mitigation and Abatement
This section intends to consolidate and evaluate established and emerging best management practices (BMP) and treatment techniques currently being used to address and manage MP. The first half of this section focuses on prevention and mitigation strategies and BMP to reduce MP from entering the environment, such as promoting a circular economy (that is, reduce, recapture, and reuse) to keep plastic materials in circulation as long as possible. The second half focuses on technologies to abate, treat, and remediate MP once it exists in the environment, whether in drinking water, surface water, groundwater, marine environments, wastewater, soil, sediment, or air (Figure 6-1). These methods and technologies are constantly evolving and are generally categorized in this section as fully demonstrated or as an emerging option. Fully demonstrated technologies are those that have been implemented or demonstrated under full-scale situations. These typically include effective, well documented treatment technologies. More information is available in Section 6.2. Emerging options may be partially demonstrated or researched and may include technologies that have been implemented at the bench- or pilot-scale. This document does not provide details about specific products or trade names nor does it serve as an endorsement for any specific technique or application strategy.
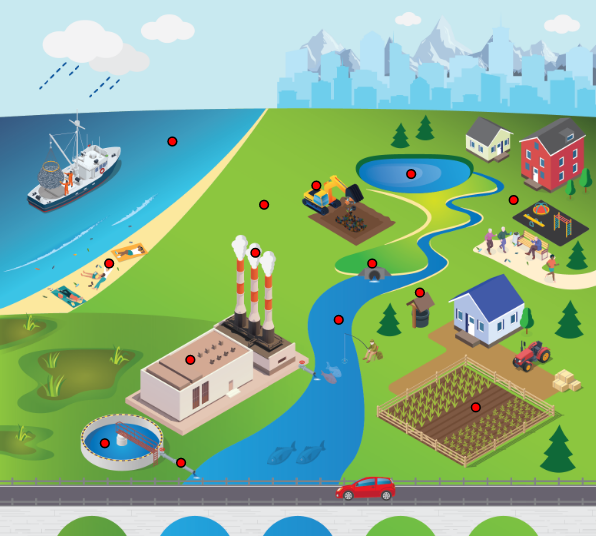
Figure 6‑1. Conceptual model for mitigating, abating and BMP for managing MP.
Source: Jonathan McDonald and ITRC MP Team
6.1 Prevention/Mitigation Strategies
The objective of this section is to present strategies for improving plastic sustainability throughout the life cycle. Strategies have been organized in four general topic areas: (1) reducing plastic packaging and increasing plastic reuse; (2) improving production efficiency, including life cycle assessments (LCA) and alternative analysis of plastic product and material substitutions to prevent regrettable solutions; (3) reducing the consumption of plastic products by reducing their appeal to consumers, especially through education; and (4) improving the disposal of wastes and advocating for recycling and recovery of plastic waste.
6.1.1 Manufacturing/Packaging
Primary and secondary MP pollution has become a major and growing global problem. The reduction of plastic packaging and increase in the reuse and recycling of certain plastics can provide some benefit to this global issue.
Table 6-1 shows the data for municipal solid waste from 1960 to 2018, relating to the total number of tons of containers and packaging generated in the waste stream, recycled, composted, combusted with energy recovery, and landfilled. As shown in Table 6-1, 13.6 percent of plastic packaging was recycled in 2018. For context, 8.7 percent of all plastics were recycled in 2018. By comparison, 31.3 percent of glass packaging, 80.9 percent of paper and paperboard containers and packaging, 34.9 percent of aluminum containers and packaging, and 73.8 percent of steel containers and packaging were recycled in 2018 (USEPA 2022a). These percentages for recycling various packaging materials seem to indicate that recycling plastic packaging is underused in the current market. Facilities should be encouraged to limit the production of plastic packaging, while increasing the percentage of materials that are recycled. The elimination of most single-use plastics, such as shopping bags and water bottles, would also help reduce the MP impact, although a major source of MP is TWP and textile fibers.
Table 6‑1. Data from 1960 to 2018 on plastic containers and packaging in municipal solid waste by weight (in thousands of U.S. tons)
| Management Pathway | 1960 | 1970 | 1980 | 1990 | 2000 | 2005 | 2010 | 2015 | 2017 | 2018 |
| Generation* | 120 | 2,090 | 3,400 | 6,900 | 11,190 | 12,420 | 13,680 | 14,680 | 14,490 | 14,530 |
| Recycled | – | – | 10 | 260 | 1,030 | 1,280 | 1,850 | 2,150 | 1,890 | 1,980 |
| Composted | – | – | – | – | – | – | – | – | – | – |
| Combustion with Energy Recovery | – | – | 70 | 1,130 | 1,960 | 2,020 | 2,090 | 2,460 | 2,470 | 2,460 |
| Landfilled | 120 | 2,090 | 3,320 | 5,510 | 8,200 | 9,120 | 9,740 | 10,070 | 10,130 | 10,090 |
A dash in the table means that data are not available.
*Generation in the waste stream before materials are recycled, composted, combusted with energy recovery, or landfilled.
Note: Plastic packaging in the table above does not include single-service plates and cups, and trash bags, both of which are classified as nondurable goods.
6.1.2 Improving Production Efficiency
The use of plastics can be reduced by (1) using alternative (for example, glass), recycled, or nonplastic biodegradable materials; (2) improving the design to reduce the amount of plastic used, extend product life, allow repair and reuse, and improve recyclability by limiting the number of polymers, additives, and mixtures; and (3) banning certain types of single-use plastics (Al-Salem, Lettieri, and Baeyens 2009, Brennholt, Heß, and Reifferscheid 2018, Browne et al. 2011, Schneider and Ragossnig 2015, Thompson et al. 2009, Prata, Silva, et al. 2019). An example of improved design is making caps inseparable from plastic bottles to increase their correct disposal (Brennholt, Heß, and Reifferscheid 2018); however, this could impair recyclability due to the presence of two polymer types. There is a demand for improvements in design, which also benefit companies by reducing requirements for raw materials, and for viable alternatives, which are still limited. Recycled plastics are more expensive than virgin plastics; however, they are beneficial at an environmental and societal level (Singh and Ruj 2015) and thus should be encouraged by voluntary (as a marketing strategy) or mandatory incorporation of a percentage of recycled materials (for example, 10% of weight), to disincentivize virgin plastic production. However, recycled content requirements should take losses in each recycling cycle into account or otherwise encourage product and material to transition to plastic alternatives (Walker and Xanthos 2018).
LCA is a tool used to assess environmental impact of a product or process from cradle to grave and identify strategic improvement opportunities such as sustainable solutions (Accorsi, Versari, and Manzini 2015, Del Borghi et al. 2021) to avoid regrettable substitutions, social, and supply chain impacts. Suggested improvements with respect to sustainability of plastics include recycling and reuse. For example, increasing recycling in PET bottles by 25%–50% would decrease their environmental impacts by 5%–230% (Prata, Silva, et al. 2019). The rapid technical evolution of additive manufacturing (AM) enables a new path to a circular economy using distributed recycling and production. Considering environmental aspects, the clear advantage of AM at the design stage (Figure 6-2) is the opportunity to produce more complex and optimized components, reducing assembly operations. Higher flexibility compared to traditional manufacturing reduces the product development time and cost, while improving human interaction and consequently improving the product development cycle (Guo and Leu 2013, Vaezi et al. 2013, Wong and Hernandez 2012).
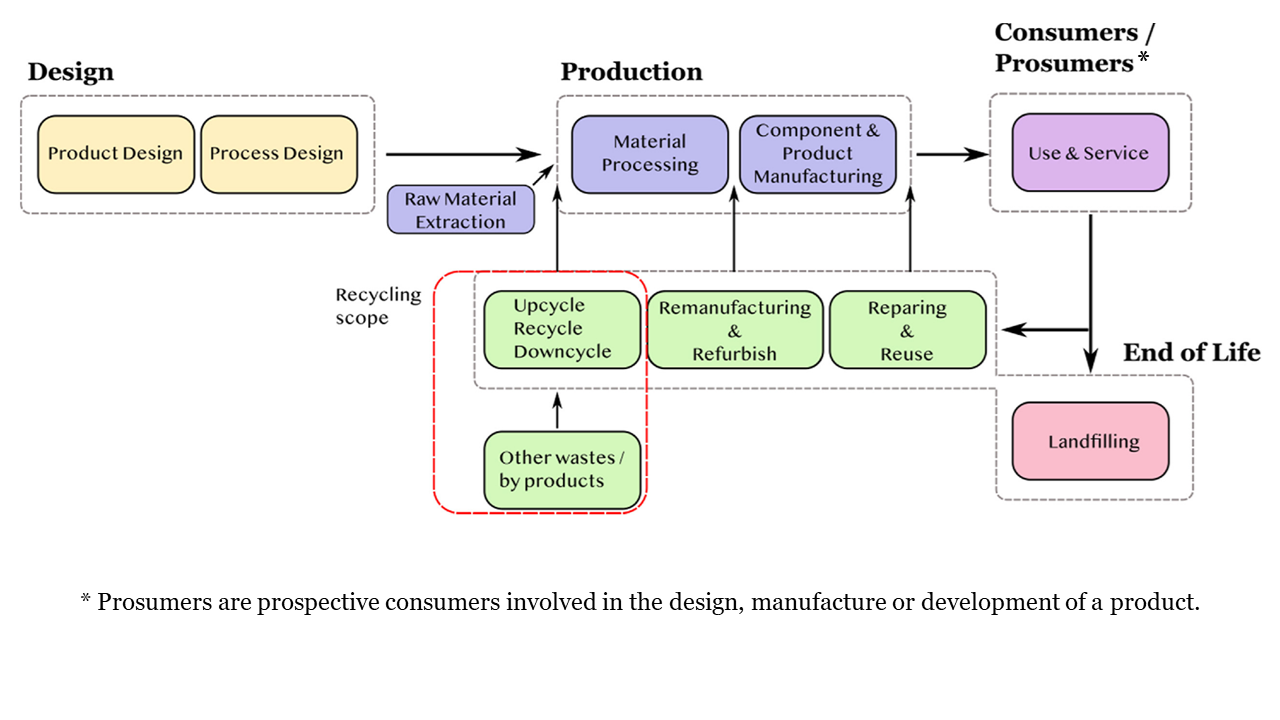
Figure 6‑2. Life cycle perspective for identifying sustainability benefits.
Source: Cruz Sanchez et al. (2020)
6.1.3 Reducing the Consumption of Plastics
The worldwide MP contamination issues can be controlled only by incorporating responsible and sustainable practices into the production, use, and waste disposition of different plastic materials. This requires collaboration between governments, corporations, and consumers to implement solutions that contribute to addressing plastic pollution, including:
- consumer education on the extent and issues associated with MP and their source materials
- behavior modifications through policy and psychological strategies
- product planning that considers product life cycle (that is, sustainable life cycle business models)
- evaluation of single-use plastics on the market for potential elimination or substitution
- development of alternative environment-friendly, reusable or recyclable materials
- establishment and expansion of reuse, recycling, and disposal facilities
- development of innovative recycling technologies with lower environmental impact
These approaches to address plastics and MP contamination are addressed in the subsections as follows.
6.1.3.1 Product Substitution
Significant reduction in plastic consumption and waste generation can be achieved through product substitution. Business and private consumers can reduce their environmental impact by assessing their use of plastic products (for example, through LCA; see Section 6.1.2) and identifying potential substitutions with other materials, such as glass or steel, paper/cardboard, or nonplastic biodegradable materials. Common plastic materials that cannot be recycled with current technology include most plastic grocery bags, cling wrap, sandwich bags, Styrofoam takeout containers, and foam packing materials. The use of reusable bags and food and beverage containers instead of disposable or lower-grade plastic materials will significantly reduce waste disposal needs and MP contribution to the environment.
The 2022 fact sheet from the Earth Day Network stated that people are using plastic bottles at the rate of about 1,200,000 plastic bottles/minute (Earth Day Network 2022b). Americans purchase about 50 billion water bottles per year, averaging about 13 bottles/month for every person in the United States (Earth Day Network 2022b).
Even in countries where high quality tap water is available, many people believe that purchasing bottled water is a healthier option, despite increasing evidence of it containing high concentrations of MP (Wong et al. 2021). Other substitution options are to order water in larger, reusable containers or to use personal water bottles for travel and outdoor activities
High quantities of plastic waste are generated from other single-use products, such as plastic bags, plates, and utensils, which can easily be substituted with reusable products.
Many toy products are made of nonrecyclable plastics, offering an opportunity to pursue reusable materials and products or otherwise educate the public about environmentally preferred alternatives.
6.1.3.2 Education and Awareness
Studies have shown that effective policy interventions in the mitigation of plastic pollution require realistic assumptions based on empirical behavioral science evidence about human decision-making (Jia et al. 2019). Heidbreder et al. (2019) conducted reviews of 187 studies that all show that people routinely use plastic, despite a pronounced awareness of the associated problems. They concluded that habits, norms, and situational factors seem to be predictors for plastic consumption behavior and that both psychological interventions and political interventions, such as bans and charges, are potentially effective. The report recommended further research on behavior-based solutions, combining interdisciplinary approaches and taking into account cultural differences.
Promoting a circular economy or implementing extended producer responsibilities can be helpful to make producers accountable, and sustainable business models have been developed on this basis (Beghetto et al. 2021, Dijkstra, Zhu, and Cheng 2021). In a review of politics and the plastics cycle, Nielsen et al. (2020) stated that political analysis and debate around plastics is concentrated at the pollution and disposal end of the plastics life cycle, with a particular emphasis on marine pollution, and that less attention has so far been given to plastics manufacture and patterns of overconsumption. They concluded that the whole plastics life cycle is political, but it has not yet been equally politicized.
Public engagement through awareness campaigns and education are important tools in building a public understanding of the issues, changing habits, and engaging participation in environmental action. Easily accessible options for learning about and participating in actions to address the impact of plastic pollution include
- Earth Day activities on April 22 and throughout April (Earth Day Network 2022a)
- UN World Environment Day on June 5 each year (United Nations 2022)
- free open online courses, lectures, and activities, for example, massive open online courses (MOOCs) on marine litter and the ocean around Nova Scotia (Al-Salem, Lettieri, and Baeyens 2009, Owens 2018)
- media such as the BBC’s Blue Planet II and National Geographic’s “Planet or Plastic”
- apps, including Marine Debris Tracker and Sea Cleaner (Jambeck and Johnsen 2015, Merlino et al. 2015)
- International Coastal Cleanup Day (third Sunday of September each year). The 17 million volunteers worldwide over the last 35 years have collected over 350 million pounds of trash (Ocean Conservancy 2022).
- organized beach cleanups, such as the Great Canadian Shoreline Cleanup (Hardesty, Good, and Wilcox 2015, Stoett 2016).
- inexpensive but valuable citizen science that could help map marine litter and demonstrate the efficacy of local management actions, such as local single-use plastic ordinances or stormwater controls (Hidalgo-Ruz and Thiel 2013, van der Velde et al. 2017)
Key elements of an education or awareness program include a discussion of MP sources, environmental and health impacts, and application of waste management hierarchy as guidance for managing plastic. Plastic materials vary in composition and quality based on product applicationand are to some extent driven by consumer demands and price. Different types of plastic have been assigned ASTM resin identification codes (ASTM 2021) that reflect material properties, and these codes appear inside a recycling symbol on most plastic materials, as shown in Table 6-2. However, the recycling symbols can be misleading because only two of the seven resin types are consistently recycled (that is, resin codes 1 and 2, PET and HDPE). Both can be shredded, cleaned, and remade into new bottles or lower-quality materials such as carpet fiber. Recycling options for other resin codes vary between service providers, so it is wise to check to optimize recycling and avoid cross-contamination with nonrecyclable items.
Table 6‑2. Resin codes for different types of plastics
| Resin Code | Plastic Type Abbreviation | Plastic Type Name | Product Examples |
 |
PET | Polyethylene terephthalate | Water and soft drink bottles, salad dressing/peanut butter containers, rope, carpet, polyester fibers |
 |
HDPE | High-density polyethylene | Milk jugs, juice bottles, freezer bags, trash bags, shampoo/detergent bottles |
 |
PVC | Polyvinyl chloride | Plumbing and construction materials, pipes, liners, cosmetic containers, commercial cling wrap, siding |
 |
LDPE | Low-density polyethylene | Squeeze bottles, regular cling wrap, trash bags, shopping bags, furniture |
 |
PP | Polypropylene | Microwave dishes, medicine bottles, straws, ice cream tubs, yogurt containers, detergent bottle caps |
 |
PS/ EPS | Polystyrene/ Expanded Polystyrene | PS—CD cases, disposable cups, egg cartons, cutlery, video cases EPS—Foam polystyrene, hot drink cups, food takeaway trays, protective packaging pellets |
Other |
POM | Acetal (Polyoxymethylene) | Fan wheels, gears, screws |
| PMMA | Acrylic (Polymethyl methacrylate) | Aquariums, fiber optics, paint | |
| ABS | Acrylonitrile butadiene styrene) | Car parts, Lego®, wheel covers | |
| PA | Nylon (Polyamide) | Air bags, clothing, thread | |
| P | Polyester | Fibers, rope | |
| PBT | Polybutylene Terephthalate | Keyboards, relays, switches | |
| PC | Polycarbonate | Eyewear, safety helmets | |
| PEEK | Polyetheretherketone | Bearings, pumps, pistons | |
| PE | Polyethylene | Mulch, housewares, toys | |
| PLA | Polylactic acid (Bioplastic) | Packaging, syringes, textiles | |
| PSU | Polysulfone | Appliance parts, filters | |
| PTFE | Polytetrafluoroethylene | Teflon® | |
| PUR, PU | Polyurethane | Adhesives, coatings, foams | |
| SAN | Styrene Acrylonitrile | Brushes, hangers, printers |
additional polymers and complex plastics are not listed.
The success of recycling plastic materials can be limited. Recycling options for plastics are generally tied to the availability of processing facilities and the product markets, which are determined by the international economics and politics of recycling, rather than vendor collection. Many countries have relied on international export of recyclables, such as to China, which in 2018 stopped accepting many types of recyclables (Wen et al. 2021). This resulted in recyclables in the United States and elsewhere being sent to landfills or incinerators, thereby turning this waste from income to expense. For example, more than 50% of Philadelphia’s recycling was being sent to an incinerator in Chester, Pennsylvania, as of January 2019 (Servedio 2020). Mixing of cross-contaminated recyclables in single-stream collection is another reason many recyclables end up in landfills (The Recycling Partnership 2020).
Plastic avoidance, minimization, or reuse have less environmental impact than recycling. Although recycling is better for the environment than disposal, it still requires energy-intensive processing and adequate recycling infrastructure that may not be immediately available. It is helpful to verify what plastic products are readily recyclable in each service area to avoid or minimize purchasing specific types of plastic. The waste management hierarchy in Figure 6-3 shows the ranking of options for handling materials, with disposal at the bottom of the triangle as the least favored option. Environmental messaging about the hierarchy typically uses the easy to-remember terminology “Refuse, Reduce, Reuse, Repurpose, Recycle” often referred to as the five R’s, instead. USEPA is now in the process of reviewing the waste hierarchy to determine if potential changes should be made based on the latest available data and information.
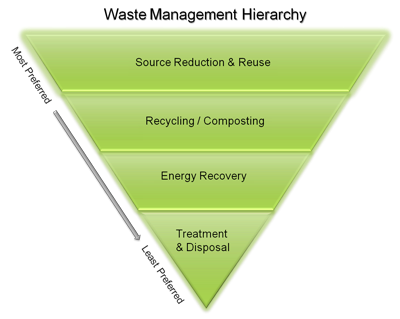
Figure 6‑3. The waste management hierarchy
Source: Adapted from (USEPA 2022g)
6.1.4 Improving the Disposal of Waste
Although some countries can get a better handle on plastics disposal through continual improvement of public awareness and expansion of sustainable waste management programs, global plastic waste and MP pollution can be addressed using a comprehensive approach that combines technology, policymaking, and advocacy through international collaboration. International efforts to fight plastic pollution initiated in recent years include the 2018 Earth Day End Plastic Pollution, and the World Environment Day Beat Plastic Pollution Campaigns, which raised public awareness and kick-started worldwide initiatives that are continuing. The UN Plastics Summit was convened in Uruguay in 2022 to develop a legally binding instrument on plastic pollution. The Environment Assembly of the United Nations Environment Programme resolution recognizes that plastic pollution includes MP (UNEP 2022a).
Significant improvements to waste disposal can be achieved only through global initiatives that involve education and buy-in on waste minimization practices; expanded collection services for reuse, recycling, and disposal; reduced production of non-reusable and nonrecyclable materials; and further improvements to recycling processes. North American and European countries typically have organized public or private waste collection services, but a large part of the world’s population does not have access to an organized collection of waste for disposal at properly designed landfills and even less for recycling, for which options are often not affordable or not available.
Realizing that most plastic waste in the environment has never been subject to organized waste collection, it is important to focus waste management improvements on organized and comprehensive collection, which ultimately serves to minimize waste. Where public waste services exist, these should be expanded to include curbside collection of household recyclables, on-demand collection of appliances and electronics, buy-back options, and drop-off centers.
There are several options for managing plastic waste:
- Reuse/repurpose ▼ Read more
- Recycling ▼ Read more
- Composting ▼ Read more
- Waste-to-energy and feedstock ▼ Read more
- Landfilling ▼ Read more
- E-waste (electronic and electric waste) Responsible Recycling (R2)-certified facilities—operation requirements applicable to plastics ▼ Read more
6.1.4.1 Improving Distribution/Storage/Transportation
Reducing or preventing releases of MP at the source, such as refining manufacturing processes to reduce and properly manage waste and implementing advanced filtration of effluent at industrial laundry facilities and water treatment plants, will provide a key step in reducing the quantity of MP entering the environment. Plastics can degrade via many different mechanisms, and improving the containment and recovery of industrial chemicals, which can cause the degradation of plastics, will aide in limiting environmental exposure. Regulating the handling of plastic pellets and other known contributors of MP to the environment will assist in limiting product degradation through physical weathering due to temperature, physical abrasion, UV exposure, and contact with chemicals that may cause degradation, thereby limiting environmental release of MP. Many states already have regulations in place for pellets (Section 5.2). By reducing the amount of degradation of plastics during storage and transport, the quantity of MP entering the environment will also be reduced (Lambert and Wagner 2018).
6.1.4.2 Stormwater Control
The pathways by which macroplastics and MP can enter the aquatic environment are numerous and include wind advection, urban creeks and stormwater runoff, and illegal dumping of plastic materials. Macroplastics break down into smaller MP as they travel along roadways, waterways, and other conveyances.
Stormwater runoff is commonly transported through municipal separate storm sewer systems (MS4s), and is then often discharged, untreated, into local water bodies. An MS4 is a conveyance or system of conveyances that is:
- owned by a state, city, town, village, or other public entity that discharges to waters of the United States
- designed or used to collect or convey stormwater (for example, storm drains, pipes, ditches)
- not a combined sewer
- not part of a sewage treatment plant or publicly owned treatment works
To prevent harmful pollutants from being washed or dumped into MS4s, certain operators are required to obtain National Pollutant Discharge Elimination System (NPDES) permits and develop stormwater management programs (SWMP). The SWMP describes the stormwater control practices that will be implemented consistent with permit requirements to minimize the discharge of pollutants from the sewer system (USEPA 2022f).
The NPDES program also requires industrial facilities and construction sites to develop, implement, update, and maintain a storm water pollution prevention plan (SWP3). The SWP3 should include best management practices (BMP) to reduce the potential for pollutants, including macroplastics and MP, to be exposed to storm water. Pollutant monitoring, which may include MP, is a requirement of an SWP3.
Although some collection methods for capturing macroplastic and other litter in stormwater have been developed (EOA 2014), methods for collecting MP in stormwater have not. One potential approach to evaluating the contribution of stormwater to MP pollution in receiving waters would be to monitor MP at the mouth of stormwater discharge locations before and after storms. Alternatively, it may be possible to develop a system to pump or monitor stormwater from the receiving waters during storm events.
Urban litter—San Francisco Bay Area monitoring studies conducted on behalf of the Bay Area Stormwater Management Agencies Association have characterized urban litter, including macroplastic items that will break down into MP in the environment. Over 150 storm drain trash capture devices were monitored between 2010 and 2011 (EOA 2014). Overall, plastic items made up 2.2%–15.1% of the trash captured by volume during the four storm events characterized, but just 0.3%–3.0% by mass, a reflection of their lightweight properties. Median household income was identified as the most consistent predictor of trash generation in a region.
Studies have quantified the MP concentrations ranging from 1.1 to 24.6 particles/L entering San Francisco Bay during wet weather events (Werbowski et al. 2021). Storm events likely play a major role in mobilizing macroplastics and MP derived from litter. Although discharge of MP to the bay via the Sacramento-San Joaquin River delta has not yet been evaluated, tributaries in the urban watersheds of the San Francisco Bay were studied (Sutton et al. 2019). A Southern California study evaluating inputs from the Los Angeles River drainage to the coastal ocean near Long Beach found that concentrations of MP increased sevenfold following a storm, from 8 pieces/m3 to 56 pieces/m3 (Moore, Lattin, and Zellers 2005).
Studies of tributaries to Chesapeake Bay (Appendix A.2) and the Great Lakes suggest that the tributaries can be a significant pathway for MP pollution (Baldwin, Corsi, and Mason 2016, Yonkos et al. 2014). Surface waters of four tributaries to Chesapeake Bay were monitored for MP monthly between July and December to assess relative loads and the influence of storms on the loads (Yonkos et al. 2014). All but one of the samples collected contained MP, ranging in concentration from <1 to >560 g/km2. Highest concentrations were associated with heavily urbanized areas and with storm events (Yonkos et al. 2014). A study of 29 Great Lakes tributaries, each sampled three or four times, found that 98% of plastic particles were small enough to be considered MP (Baldwin, Corsi, and Mason 2016). Fragments, films, foams, and pellets were found at higher levels in tributaries draining urban watersheds, and during conditions leading to runoff, such as rainfall.
A combination of structural and nonstructural BMPs and stormwater controls can lessen the amount of macroplastics and MP introduced into waterways. Nonstructural BMPs are preventive actions that involve management and source controls, such as creation of buffers along water bodies, minimization of impervious surfaces, and reduction of disturbance to soils and vegetation. Structural BMPs include storage practices such as wet ponds and extended-detention outlet structures; filtration practices such as grassed swales, sand filters, and filter strips; and infiltration practices such as infiltration trenches and infiltration basins (USEPA 2005). It must be considered that MP removed by the structural BMPs listed above may create soil and groundwater contamination. Other structural BMP that are effective in removing macroplastics are booms and trash capture devices installed in waterways.
Source reduction is also effective in reducing plastic pollution in stormwater. Trash surveys in California found a significant decrease in plastic bags, likely due to the implementation of a statewide bag ban in 2016 (McLaughlin et al. 2022). Other nonstructural BMPs may include enforcement of litter ordinances, employee training regarding good housekeeping practices at commercial facilities, and container deposit laws. “Bottle bills” are a type of container deposit law using a deposit-refund system that encourages consumers to return empty beverage containers for a refund. Studies conducted pre- and post-bottle bills show that container deposit legislation reduces beverage container litter by 69% to 84%, and reduces total litter by 30% to 65% (BottleBill.org 2022).
6.2 Abatement/Treatment
Preventing MP from entering the environment through source reduction techniques is the best first line of action; however, where MP have already entered the environment, treatment technologies are being developed to capture and remove MP from environmental media. The information presented in the following sections reflects the availability of performance results published, presented, or posted to the internet. Those technologies that have been implemented in the field at multiple sites, by multiple parties, and have peer-reviewed documentation of performance are discussed in Section 6.2.1 (water), Section 6.2.2 (soil), and Section 6.2.3 (sediment) below.
6.2.1 Water
Although acceptable levels of MP in water and the understanding of MP impact on human health and the environment are still in development, establishing treatment criteria and objectives is challenging. With limited regulations or guidance available at this time, it is up to decision-makers and experts to identify and select the most promising treatment technologies to achieve the desired water quality. Table 6-3 provides a summary of potential treatment technologies for removing MP from water.
Table 6‑3. Treatment technologies for MP in water
| Treatment Category | Treatment Technology | Media | Advantages/ Efficiencies | References |
| Biological | Field Implemented (for Select Media)/General Remediation Technology | |||
| Rain garden (bioretention cell) | Stormwater | Up to 98% MP removal efficiency | Werbowski et al. (2021) | |
| Developing Technology or at Lab Scale | ||||
| Biodegradation | Surface water, groundwater, wastewater, marine, soil, sediments | 75–99% MP removal efficiency
A consortium of organisms can be used as a treatment strategy |
Gan and Zhang (2019), Han et al. (2017), Hu et al. (2021), Pathak and Navneet (2017) | |
| Chemical | Developing Technology or at Lab Scale | |||
| Chemical degradation (oxidation, hydrolysis) | Surface water | Up to 56% MP weight loss for Fenton-like system
Builds off treatment technologies used for other |
Hu et al. (2021) | |
| Electrochemical oxidation | Surface water, groundwater, marine, wastewater, soil | 58% MP removal efficiency, and up to 86.8% with an additional oxidant
Quick treatment time; |
Kiendrebeogo et al. (2021) | |
| Physical | Field Implemented (for Select Media)/General Remediation Technology | |||
| Classic coagulation and agglomeration | Drinking water | 70–83% MP removal efficiency for drinking water (combined coagulation/ flocculation, sedimentation or flotation as well as filtration using sand and activated carbon treatment) Can sequentially |
Pivokonsky et al. (2018) | |
| Filtration- membranes (reverse osmosis, ultrafiltration, nanofiltration, etc.) | Drinking water, groundwater, surface water | 99.9% MP removal efficiency for membrane bioreactor in tertiary step of treatment sequence
Can |
Ali et al. (2021), Hu et al. (2021) | |
| Developing Technology or at Lab Scale | ||||
| Sedimentation/flotation | Drinking water, groundwater, surface water | 61% MP removal efficiency using iron and aluminum-based salt coagulants
Can sequentially combine |
Padervand et al. (2020) | |
| Electrocoagulation | Drinking water, wastewater, groundwater | More than 90% MP removal efficiency, and up to 99.2% at pH 7.5
Eco-friendly and efficient, |
Perren, Wojtasik, and Cai (2018) | |
| Magnetic extraction | Surface water, groundwater | 78%–84% removal efficiency for medium-sized MP and 92% of small MP
Eco-friendly and efficient, |
Tang et al. (2021) | |
| Acoustic focusing | Surface water | Allows for directed application of removal techniques | Akiyama et al. (2020) | |
| Physical degradation | Surface water | Allows transformation of MP to facilitate application of treatment technologies | Lambert and Wagner (2018) | |
| Photodegradation (UV light) | Surface water, wastewater | 100% MP removal efficiency for photocatalysis
Transformation of some plastics (PE, PP, PVC) into |
Hu et al. (2021) | |
| Thermal (that is, pyrolysis and gasification) | Surface water, soil | 54% in MP weight loss for catalytic advanced oxidation process with hydrothermal hydrolysis | Hu et al. (2021) | |
| Physical/ Chemical | Developing Technology or at Lab Scale | |||
| Adsorption | Surface water, groundwater | 100% MP removal efficiency at pH 4, 37% at pH 9 of nanoscale plastics (layered double hydroxide [LDH]) MP have high sorption capacity |
Hu et al. (2021) | |
| WWTP/DWTP Treatment Sequencing | Field Implemented (for Select Media)/General Remediation Technology | |||
| Primary treatment (grit chamber, grease removal, primary settling tank) | Wastewater | 60–90% MP removal efficiency
Treatment processes/equipment used with most conventional wastewater |
Ali et al. (2021) | |
| Primary treatment (screening/grit removal and sedimentation) | Wastewater | Average 31.8% (stage-wise & overall) | Ziajahromi et al. (2017) | |
| Primary treatment (aerated grit chambers and oxidation ditch) | Wastewater /sewage treatment plant | 16.5% (stage-wise and overall) | Lv et al. (2019) | |
| Primary treatment (aerated grit chamber, primary sedimentation tank) | Wastewater | 58.84 ± 8.05% (stage-wise & overall) | Yang et al. (2019) | |
| Secondary aeration or biological treatment, flocculation or sedimentation, and UV disinfection or dechlorination |
Wastewater | Average 68% (stage-wise), average 78.1% (overall) | Ziajahromi et al. (2017) | |
| Secondary treatment (activated sludge reactors, bioreactors, secondary clarifiers) | Wastewater, drinking water | 50–98% MP removal efficiency
Treatment processes/equipment used with most conventional wastewater |
Ali et al. (2021) | |
| Tertiary treatment (filtration, coagulation/flocculation, ozone) | Wastewater, drinking water | 50–98% MP removal efficiency
Treatment processes/equipment well established in the market |
Ali et al. (2021) | |
| Coagulation-flocculation and sand filtration | Drinking water | 70% (overall) | Pivokonsky et al. (2018) | |
| Coagulation-flocculation, sedimentation, sand and granular activated carbon | Drinking water | 81% (overall) | Pivokonsky et al. (2018) | |
| Coagulation-flocculation, sedimentation, flotation, sand and granular activated carbon filtration | Drinking water | 83% (overall) | Pivokonsky et al. (2018) | |
Refer to Section 2.3 for a description of the distribution, fate, and transport of MP in water.
6.2.1.1 Drinking Water
Treatment through conventional process in drinking water treatment plant (DWTP): Treatment plants for drinking water frequently draw water from different water sources, including surface water, groundwater, and seawater for treatment and these sources of water are subject to MP contamination (Collivignarelli et al. 2018). Various studies have reported the presence of MP in both raw and treated drinking water, tap water, and bottled water (Nikiema et al. 2020). Drinking water treatment is similar to wastewater treatment except that the secondary treatment for removal of organic matter is absent. The reason is that raw water feeding DWTPs usually contains significantly less organic load and is better in quality compared to municipal and industrial wastewater. Like wastewater treatment, drinking water treatment starts with screening and grit removal, followed by addition of alum to the raw water for coagulation and flocculation (Figure 6-4). The mixture subsequently enters the sedimentation tanks, where heavy floc particles settle to the bottom, akin to wastewater treatment. Unlike wastewater treatment, the water goes through filtration instead of secondary treatment, and the filtration unit is typically a sand filter with an optional activated carbon filter. Most studies conducted for DWTPs do not distinguish the efficiency of MP removal at different stages of treatment, probably because the stages of treatment are not as clearly defined as WWTPs. Three drinking water treatment plants in the Czech Republic demonstrated an average MP (>100 μm to 1 μm) removal of 70%–83% with water treatment comprising coagulation/flocculation, sedimentation or flotation, as well as filtration using sand and activated carbon. The combination of three processes reported the highest efficiency (Pivokonsky et al. 2018). Further studies suggest that coagulation and membrane filtration seem to be more promising (Nikiema et al. 2020).
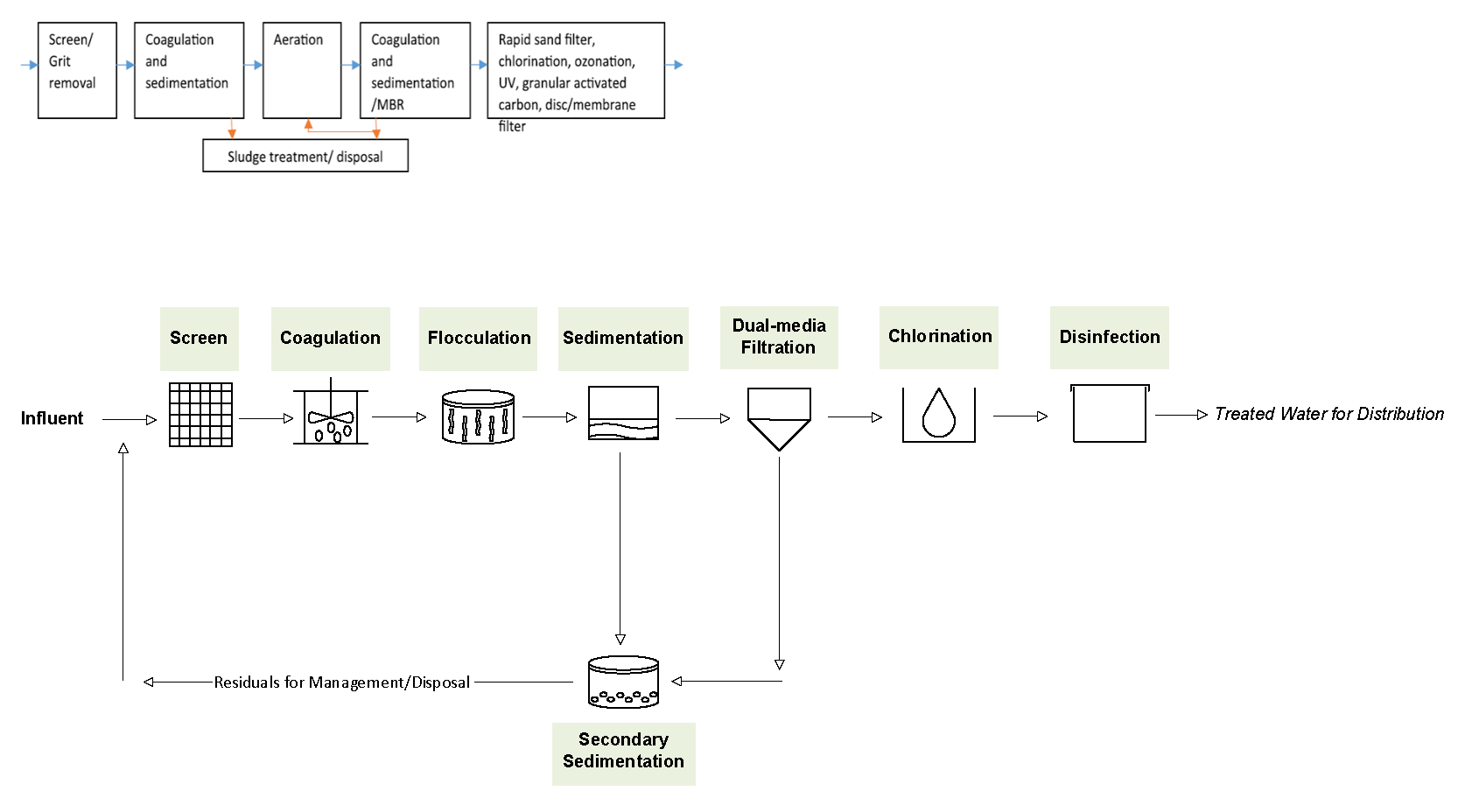
Figure 6‑4. Stages of drinking water treatment.
Source: Arcadis
The treatment of MP in DWTPs depends on the MP concentrations, sizes, and DWTP configuration—one or a combination of more than one process (Pivokonsky et al. 2018). Further studies reported that the treatment also depends on specifics of the treatment process, including dosage and types of flocculants and MP (Ma, Xue, Ding, et al. 2019, Ma, Xue, Hu, et al. 2019). For example, the Al-based coagulant showed better performance than Fe-based salt, but the required dose for both coagulants was extremely high compared to doses commonly applied for coagulation in drinking water treatment (Nikiema et al. 2020, Novotna et al. 2019).
In addition, the treatment efficiency in DWTPs may also be rated to other factors, such as the source of raw water, etc. (Mintenig et al. 2019). As mentioned in Section 2.3.3, groundwater appears to contain less MP compared to other water sources; therefore, the treatment efficiency and cost in DWTPs using groundwater as raw water are expected to be different from those using other types of raw water. Drinking water distribution and final consumption may also reintroduce MP into delivered water due to abrasives of the plastic delivery materials because the delivery material (for example, pipes and containers, etc.) in households is often made of plastic (Mintenig et al. 2019, WHO 2019).
Degradation of plastic depends on the physico-chemical properties of the polymers and environmental conditions, such as weathering, temperature, irradiation, and pH (Uheida et al. 2021). Various degradation technologies (abiotic and biotic methods) of plastic treatment have been studied in different environments, such as the marine environment, marine sediment, air, soil, etc. However, most of the current research on the environmental degradation of plastics has been focused on the early stage. Degradation in the freshwater environment received even less attention. It was suggested that future research should further identify the key environmental parameters and properties of plastics affecting the degradation to facilitate the treatment and removal of plastics (Zhang, Hamidian, et al. 2021).
Emerging technologies of MP removal in water treatment: There is a constant search for new technologies to remove MP from water and water treatment wastes. A recent study demonstrated that an innovative method using acoustic waves was applicable to collect MP in water. Model MP, including PS microparticles and small fibers of PA and PET, were studied, and the results indicate about 56% of MP removed in pure water and 58% in seawater (Akiyama et al. 2020). Although the study is ongoing, the available results suggest this technology has the potential to be used in the treatment in other aqueous media (for example, surface water, wastewater, etc.).
Photodegradation/Photocatalysis: Photocatalysis is an eco-friendly technique that is capable of degrading a wide range of organic pollutants for water treatment (Koe et al. 2020). The mechanism of photocatalysis is to convert photon energy to chemical energy and generate different reactive species based on the use of suitable wide bandgap metal oxide semiconductor materials such as titanium dioxide, zinc oxide, ferrous oxide, etc., interacting with light sources. These reactive species initiate the polymer degradation process, leading to chain scission and degradation. Uheida et al. (2021) confirmed that visible light irradiation of zinc oxide nanorods reduced the average volume by 65% for PP particles in the size of 154.8± 1.4 µm in a flow water system via chain scissions mechanism. The by-products generated after photodegradation may be considered to have low toxicity in humans and the aquatic environment. Although only the early degradation stage was investigated, photodegradation has great potential for use in both drinking water and wastewater treatment systems due to its advantages, such as the unitization of sunlight as a clean energy source, low cost, and generation of harmless by-products (Uheida et al. 2021).
Electrocoagulation: Electrocoagulation uses electrochemical reactions instead of merely chemicals or microbes to induce coagulation, hence is more cost effective (Garcia-Segura et al. 2017). Hydroxides of Fe3 + or Al3 + are produced during the electrochemical reaction, which upon colliding with the pollutant particles, form micro-flocs. In an experiment by Perren, Wojtasik, and Cai (2018) to investigate the effectiveness of electrocoagulation in removing MP, removal efficiency of more than 90% was reported and up to 99.2% was achieved when the pH was 7.5.
Magnetic extraction: Magnetic extraction offers another potential method of water treatment to remove MP. It uses Fe nanoparticles coated with hexadecyltrimethoxysilane as magnetic seeds for magnetic extraction of MP from water (Grbic et al. 2019). This method was able to recover 84% and 78% of medium-sized MP (200–1,000 μm) from fresh water and sediment, respectively, and extracted 92% of small (<20 μm) PE and PS MP from seawater. Its ability to recover MP from sediment, though at lower efficiency due to obstructed movement of Fe nanoparticles by soil, implies its potential applicability for MP removal from biosolids (Grbic et al. 2019).
Studies on the treatment and removal of MP in drinking water, including data about the removal efficiency, are limited (Nikiema et al. 2020). Future studies on more accurate identification technologies would be beneficial to better understand the removal efficiency (Mintenig et al. 2019). During the treatment process, MP may interact with other chemicals, such as chlorine or chlorine compounds, organic compounds, etc. (Kelkar et al. 2019, Napper et al. 2015). The interactions may affect the treatment efficiency and the evaluation of MP treatment. However, the understanding of these interactions is another knowledge gap of understanding MP treatment (Nikiema et al. 2020).
The cost of plastics removal drinking water source was estimated to vary based on the source of water used, as mentioned above. The annual operation and maintenance cost of using DWTPs is approximately $1.50/m3 (Heberling et al. 2017, Nikiema et al. 2020, Plappally and Lienhard 2013)
Management of water treatment wastes such as biosolids, membranes, and filtrate concentrate is discussed in Section 6.2.1.7.
6.2.1.2 Surface Water
Most studies have focused on MP in marine systems, with <4% of publications concerning freshwater systems (Lambert and Wagner 2018). Oceanic plastic debris mainly originates from the land, where innumerable plastic particles and fibers accumulate (Li et al. 2018). As shown in Figure 2-3, MP enter freshwater environments through various routes, including discharge of domestic wastewater (Murphy et al. 2016), application of biosolids from wastewater treatment plants in agriculture (Nizzetto, Futter, and Langaas 2016), landfill leachate (He et al. 2019), industrial and wastewater treatment plant effluent (Hu et al. 2021), surface runoff (little is known about transport by runoff), and atmospheric deposition (Dris et al. 2016). There are currently limited treatment options for MP in surface water, with research in this field being in its relative infancy. A few examples exist of surface water–specific pilot-scale treatment implementation; however, these in situ systems target low-density MP (those near the water surface), and macroplastics that may degrade and contribute to MP in the environment (ESE Magazine 2021). Large-scale implementation of removal technologies for surface water has not yet been performed. Due to the limited nature of currently implementable technologies, the best method for managing MP in surface water is preventing them from entering the water cycle, as described in many subsections within Prevention/Mitigation Strategies, Section 6.1.
The Yangtze estuary in China is considered to be the largest contributor of plastic to the ocean in global modeling studies (Lebreton et al. 2017), and thus it has raised the level of concern. Li, Lu, et al. (2020) showed that MP are much more abundant in surface water in the Yangtze estuary than in the inland rivers, while sediment contamination levels were similar in the estuary and the inland rivers. They also found compositional differences in MP between the inland rivers and estuary, especially in sediments. In addition, significant density differences between surface water and sediments verified the diverse vertical transport of MP in inland rivers and the estuary, where resuspension of sediments directly influenced redistribution in both phases.
The use of constructed wetlands for MP removal from surface waters has shown promise as an economical and efficient removal technology in both field- and lab-scale implementation. When vegetated strips or transitions in riparian buffer areas are situated parallel to the banks of water bodies, treatment wetlands can be designed such that suspended solids settle out within the vegetation. Constructed wetlands provide a primary management option for MP moved by surface waters between pollutant sources and receiving waters and can be sequenced with several mitigation technologies in a treatment train process to provide removal rates up to 100% (Chen et al. 2021, Hassan et al. 2021, Wang, Hernández-Crespo, et al. 2021). Constructed wetlands can also be engineered to use specific flow regimes to support the removal of low-, neutral-, and high-density MP. These constructed wetland flow regimes include vertical subsurface, horizontal subsurface, and horizontal surface flow (Hassan et al. 2021, Wang, Hernández-Crespo, et al. 2021).
To limit the remobilization of MP during large eroding events such as storm or seasonal runoff causing an increase in flow rate, the MP‑containing sediment in the settling basins requires periodic cleanings and proper disposal. Likewise, as the riparian flora change over time due to the life cycle of the flora or changing environmental conditions that restrict or no longer support growth, the MP bound within the root structure may remobilize and any MP uptake by the flora will be reduced or eliminated (Hassan et al. 2021). As an addition to physical capture and uptake through flora in constructed wetlands, MP can also undergo degradation via microorganisms (Chen et al. 2021, Wang, Hernández-Crespo, et al. 2021)
Current research in surface water technologies for MP removal have many parallels to drinking water treatment (Section 6.2.1.1), pump and treat technologies applicable to groundwater treatment (Section 6.2.1.3), and WWTP technologies (Section 6.2.1.5).
Many possible remediation approaches are under examination in lab-scale implementation as provided in detail below.
Biodegradation by organisms: There are mainly four stages of biodegradation: (1) fragmentation of plastics via biofilms; (2) depolymerization of plastic fragments through extracellular enzymes released from microorganisms; (3) small, depolymerized molecules penetrate microorganism cells; and (4) mineralization of small molecules via intracellular enzymes (Fojt et al. 2020, Hu et al. 2021). Bacterial degradation, in conjunction with enzymes, has been observed in Kocuria palustris M16 and Rhodococcus sp. 36 from the Actinobacteria class and various Bacillus strains, with rates of 1%–10% weight loss of MP observed between 30 days and one year. Hu et al. (2021) indicated that fungi have proven effective on various plastic substrates with increased efficacy observed with the addition of pro-oxidants. Fungi are highly adaptable to plastic substrates, including LDPE, HDPE, PP, PVC, PET, PA, P, PU, and polystyrene sulfonate.The green algae Chlorella vulgaris is capable of using intermediary MP degradation products as a carbon growth source.
Adsorption: As stated in Section 6.2.1.1, membrane bioreactors have been shown to decrease the number of MP polymers in effluent, indicating a high sorption capacity allowing for the capture of MP (Hu et al. 2021). Zinc-aluminum layered double hydroxides (LDH) have been observed to have high sorption capacity for MP removal (Tang et al. 2021). MP have been observed to adsorb onto green microalgae.
Magnetic extraction: This technology uses adsorption of MP to metal surfaces (Hu et al. 2021, Tang et al. 2021). Iron oxide and magnesium hydroxides have been developed for the removal of suspended MP. Magnetic carbon nanotubes have been developed as absorbent substrates for the removal of MP. Magnetic nanoparticle composites, based on a polyoxometalate ionic liquid, have been used to remove organic MP.
Acoustic focusing: Piezovibrations have been identified in research to collect MP and MP fibers (Akiyama et al. 2020, Perera and Piyasena 2022). Acoustic focusing technology is under investigation as a method to isolate MP in aqueous samples. Early findings indicate that this technique is promising for MP isolation, with some specific considerations needed for size and density of the MP (Perera and Piyasena 2022)
6.2.1.3 Groundwater
The remediation of MP in groundwater is still in the early stages of development. Treatment of groundwater impacted by MP can be performed ex situ via pump and treat systems, and research is ongoing to develop in situ treatment technologies.
Ex situ treatment: Groundwater impacted by MP can be treated ex situ via pump and treat systems. Groundwater is pumped from wells to an aboveground treatment system that removes the contaminants. Groundwater used as drinking water may be treated with the drinking water treatment methods described in Section 6.2.1.1, such as coagulation, flocculation, sedimentation/flotation, filtration (that is, sand, granular activated carbon), and membrane processes. Other ex situ treatment technologies in development include:
- treatment trains, such as the combination of granular activated carbon filtration, high doses of FeCl3 6H2O/AlCl3 6H2O coagulants and ultrafiltration through polyvinylidene fluoride membranes (Novotna et al. 2019)
- electrocoagulation, in which the liberation of metal ions from electrodes will form coagulants that destabilize the surface of MP (Perren, Wojtasik, and Cai 2018)
- magnetic extraction, through application of magnetic carbon nanotubes that will adhere and cluster MP for removal (Tang et al. 2021)
Certain technologies, such as membrane processes, generate secondary wastes as a byproduct of treatment. Waste management of membranes and membrane concentrate is described in Section 6.2.1.6.
In situ remediation: Some technologies have been identified as potential in situ technological approaches that could be applied for groundwater remediation of MP, although these technologies have not yet been tested under the target circumstances. These potential technologies include:
- in situ biodegradation—use of microorganisms or fungi capable of degrading plastic polymers polymers (Gan and Zhang 2019, Han et al. 2017, Pathak and Navneet 2017)
- in situ biostimulation—the application of growth supplements, fertilizers, natural surfactants, and nanoparticles, along with the optimization of environmental requirements, to promote in situ plastic bioremediation (Patrício Silva 2021).
6.2.1.4 Marine
The marine environment, being the end receiver of the plastic, has accumulated the majority of the MP released from various sources. Plastics in the form of macro-, meso-, micro-, and nanoplastics are common in the marine environment. The concentration of NP was estimated to be beyond 1014 times that of MP in marine environments (Besseling et al. 2019). Surface water treatment techniques can also be applied for marine water treatment, but the impact of high salinity should be considered. Remediation approaches are unlikely to play a significant role in the reduction of MP in marine waters in most cases; however, unique technologies and destructive paths for the marine environment are discussed below.
Biodegradation: A marine fungus, Zalerion maritimum, is capable of using PE, resulting in the decrease in both mass and size of the pellets (Paço et al. 2017). These results indicate that this naturally occurring fungus may actively contribute to the biodegradation of MP, requiring minimum nutrients in the marine environment. The transformation of PE was conducted in a laboratory and was not tested in the field.
Electrochemical oxidation: Electrochemical oxidation (EO) has been used as a wastewater treatment method to remove organic contaminants for decades. The most general layout comprises two electrodes, operating as anode and cathode, connected to a power source. When an energy input and sufficient supporting electrolyte are provided to the system, strong oxidizing species are formed, which interact with the contaminants and degrade them. During electrolysis, electron-rich organics are directly oxidized on the anode surface or indirectly oxidized by oxidants that are generated from the anode. The marine environment contains high levels of salts as the electrolyte, resulting in favorable conditions for EO treatment. Therefore, EO can be particularly effective for MP and NP destruction. EO has demonstrated effectiveness in reducing the MP size and mass and mineralizing NP (Oliveira et al. 2022). The obtained results revealed that the EO process using a boron-doped diamond (BDD) anode degrades 58 ± 21% of MP in 1 hour (Kiendrebeogo et al. 2021). The combination of dynamic light scattering, scanning electron microscopy, total organic carbon, and Fourier transform infrared spectroscopy results suggested that the MP did not break into smaller particles, but that they degrade directly into gaseous products. When an oxidant, such as peroxide, was applied to the EO system, the NP degradation efficiency increased up to 86.8% (Kiendrebeogo et al. 2021). EO technology, which shows promise to destruct MP and NP, has not been applied to large-scale treatment systems to date
6.2.1.5 Wastewater
This section focuses on the removal of MP from wastewater at WWTPs. Ali et al. (2021) performed a critical review of literature involving MP and NP in WWTP published between January 1990 and March 2021. This review included 176 MP reports and 67 NP reports. The research has focused primarily on MP, as very little focus has been on the status of NP (<0.1 mm) in WWTPs. The below summary is taken from this review and is shown conceptually in Figure 6-5.
Studies have shown that WWTPs play an important role in the discharge of MP to receiving water bodies, despite conventional WWTPs being able to remove >90% of the MP from sewage, as the MP in the treated effluent is still a considerable quantity. In addition, 50%–85% of the MP could be retained in the sewage biosolids, which is widely applied to farmland and can contribute to soil pollution. The amount of destruction of MP in WWTPs has been considered negligible. No specific treatment technologies have yet been developed and applied specifically for WWTPs for the elimination of MP and NP from sewage outside of the conventional treatment processes, such as grit removal, skimming, sedimentation, filtration, and bioreactors.
MP and NP in wastewater come from numerous sources, including household and industrial discharge. The primary types of MP and NP are fibers, fragments, beads, foams, films, sheets, granules, and pellets. In terms of shape, fibers accounted for the highest proportion (25%–75%) of MP found in wastewater, followed by fragments (15%–65%), films (8%–24%), microbeads (5%–12%), foam (1%–3%), and others (1%–2%). The large number of fibers was attributed to domestic washing and textile manufacturing; foam and film particles were attributed to erosion of plastic bags and packaging products; and pellet and microbeads were attributed to personal care products. The size of MP affected removal, with the larger size particles (>500 mm) in lower concentrations in the effluent than the influent. No information was available on NP removal. Over 45 kinds of MP polymers were detected: PS (20%–90%), P (40%–75%), PE (5%–60%), PP (2%–40%), PET (3%–35%), PA (2%–35%), and acrylate (2%–28%) were the most commonly detected in WWTP influent (Ali et al. 2021).
Removal efficiencies of MP in different stages of WWTPs were estimated based on published research papers between 2015 and 2021 and are shown in Figure 6-5. The different stages include preliminary and primary treatment (for example, grit chamber, grease removal, primary settling tank), secondary treatment (for example, activated sludge reactors, bioreactors, secondary clarifiers), and tertiary treatment (filtration, coagulation/flocculation, ozone).
Figure 6-5 shows a conceptual estimation of MP removal and retention in different stages of WWTPs.As shown on this figure,removal efficiencies in the preliminary and primary treatment average 50%–85%, with 30%–60% removal in the preliminary treatment and 45%–75% removal in primary treatment. The primary removal mechanisms are entrapment of MP in flocs of fat, oil, and grease, and gravity separation. The removal efficiencies were a function of size (MP >2.5 mm could be retained by fine screens) and by density (>1.5 g/L could be removed in the grit chamber and <1.0 g/L (common of microbeads) could be removed by skimming). The removal of particles in the grit chambers was a function of settling time, which needed to be less than the travel distance divided by the water velocity. Studies also showed that the combination of flocculation and primary clarification could remove >90% of MP (Ali et al. 2021). Note that the removal efficiencies of these treatment units depend on the influent concentrations of MP to the unit.
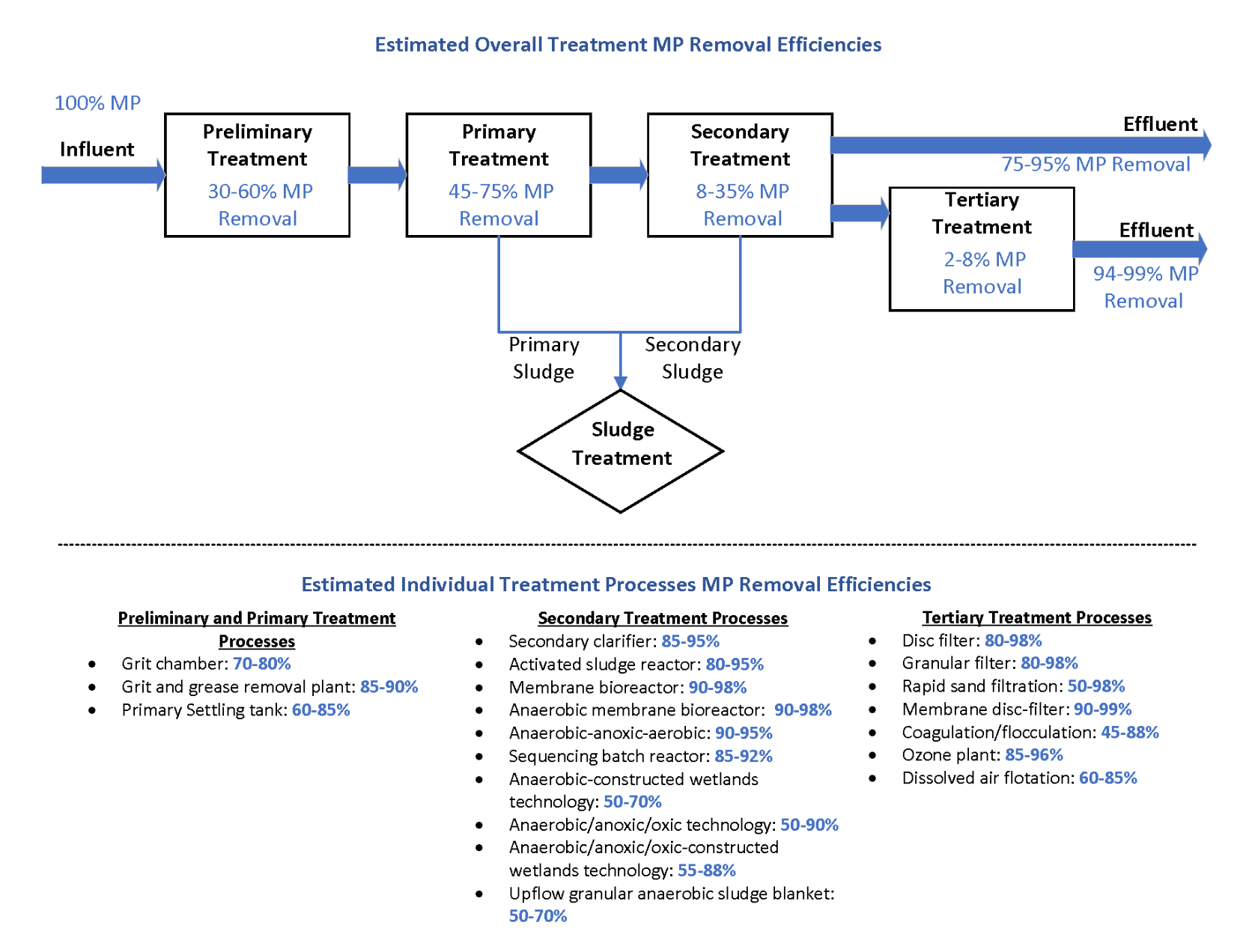
Figure 6‑5. A conceptual estimation of MP removal and retention in different stages of WWTPs
Source: Renee Lu, modified from Ali et al. 2021
Removal efficiencies in secondary treatment averaged 8%–35%, though individual removal efficiencies could be as high as 95%. Activated sludge could remove 80%–95% of MP with the main removal mechanism being adsorption and aggregation with biosolids flocs; degradation by microorganisms was considered negligible. Biofilm reactors, such as trickling filters, rotating biological contactors, and biological fluidized beds, could remove 50%–80% of the MP through adsorption or fixation and then settlement in the biosolids once the biofilm growth falls away. A membrane bioreactor (MBR) is a variant of the activated biosolids process that integrates a membrane process such as microfiltration or ultrafiltration. MBR can remove 99.9% of the MP because the membrane used employ microfilters or ultrafilters (Ali et al. 2021).
Removal efficiencies in tertiary treatment averaged 2%–8%, relative to the primary effluent, with individual removal efficiencies ranging from 50% to 99%. During membrane treatment, MP could be adsorbed within and onto the pores, though biofouling could be an issued. Rapid sand filters could result in the splintering of MP into smaller particles that may not be removed. Coagulation/flocculation could remove 45%–88% of MP from sewage, with better results obtained from aluminum-based coagulants than iron-based coagulants (Ali et al. 2021).
Overall, approximately 75%–95% of MP removal was observed for a combination of primary-secondary treatment and 94%–99% of MP removal was observed for a combination of primary-secondary-tertiary treatment processes (Figure 6-5).
6.2.1.6 Emerging Technologies for MP Removal in Wastewater
Biodegradation: All of the conventional WWTP technologies listed above physically separate MP from the wastewater and generally do not degrade MP. As discussed above, between 75% and 99% of MP can be removed by WWTP unit processes, depending on the type of WWTP, which means that between 1% and 25% of all MP remains in the WWTP’s treated effluent and is discharged to the environment, most likely a surface water. In addition, WWTPs fragment 80% of MP into NPs, which can increase the number of plastic particles by 10 times (Enfrin, Dumée, and Lee 2019). To date, very limited studies have been conducted on the degradation of MP in water. Generally, there are three types of degradation: microbial degradation, advanced oxidation processes, and thermal treatment outside of removal in WWTPs (Hu et al. 2021).
In biodegradation, microorganisms such as bacteria can use the MP as the carbon or nitrogen source to survive and reproduce. A comprehensive review by Roager and Sonnenschein (2019) found that bacteria were capable of degrading MP at very slow rates (for example, between 30 days and 1 year). One exception was Ideonella sakaienis 201-F6, which could degrade PET after 6 weeks at 30ºC (Yoshida et al. 2016). Effective degradation of other plastics, such as PE, PP, PVC, PS, PUR and PA have also been shown (Amaral-Zettler, Zettler, and Mincer 2020). Fungi are also capable of using MP as a carbon source. Compared with bacteria, fungi were reported to have better performance in PE degradation (Muhonja et al. 2018).
Initial efforts in advanced oxidation processes focused on the photocatalytic degradation of MP; a couple weeks was required to meet acceptable degradation efficiencies (Phonsy et al. 2015).
Electrochemical oxidation (EO): Recently, research has been done using EO to degrade persistent pollutants such as pharmaceuticals, pesticides, dyes, and petrochemicals (Zhuo et al. 2016). EO is based on the in situ generation of oxidizing radicals (for example, hydroxyls) by direct and indirect electrochemical processes. The radicals degrade MP by breaking their polymeric bonds into nontoxic molecules (for example, water, carbon dioxide). A recent study by Kiendrebeogo et al. (2021) demonstrated that 89% +/- 8% mineralization of MP (PS) could be achieved in 6 hours using Na2SO4 as a supporting electrolyte and a 9-amp current at an estimated cost of $68.5/m3.
Membrane technology: Membrane technology is used on its own in the treatment of water with high total dissolved solids or in combination with secondary treatment processes in wastewater treatment. It is also frequently employed in tertiary treatment of secondary effluents of WWTPs. Reverse osmosis (RO) has been used in an Australian WWTP for treatment of tertiary effluent and has managed to reduce MP from 0.28 to 0.21/L of effluent (Ziajahromi et al. 2017). This is still far above the 0.005 MP/L reported by Talvitie et al. (2017) for MBR despite smaller pore size of the RO membrane than that of the ultrafiltration membrane of the bioreactor. Irregularities of pores with presence of larger pores on the membrane and other defects could cause the escape of MP through the RO membrane (Yang, Shi, et al. 2015). There has been interest in improving the efficiency and cost-effectiveness of membrane technology in water treatment. Beljanski et al. (2016) developed and tested a low-cost bench-scale gravity-powered filtration system to identify the optimal filter type, filter angle, and pressure for removal of MP. A condition similar to a WWTP was simulated for the testing. Two types of filters were compared—a 2-D filter of 80 μm screen and a thicker multilayer 3-D filter of the same screen size. The test demonstrated faster clogging of the 2-D filter but better removal of MP during backwashing compared to the 3-D filter, which tended to trap MP. Backwashing of the 3-D filter at a pressure of 1.68 and 3.68 kPa could remove 95%–100% of microfibers, though lower pressure required longer backwashing duration (Beljanski et al. 2016).
The American Society of Civil Engineers gives U.S. infrastructure relating to wastewater treatment an overall D+ grade (ASCE 2021). It is inevitable that significant expenditures are necessary to meet the conventional requirements for U.S. wastewater infrastructure. The issues associated with plastics in our water and land resources can be incorporated in required infrastructure upgrades. Wastewater infrastructure in general has a lifespan of about 50 years and a residential system has about half that. Many of the existing plants and systems are operating past that. None of these plants were built to consider MP treatment
6.2.1.7 Management of Water Treatment Wastes to Reduce/Remove MP
Sludge. Sludge is formed during settlement of floc particles after coagulants are added to the raw water or wastewater to induce floc formation. Flocs subsequently settle and accumulate as sludge (Ali et al. 2021, Mahon et al. 2017). An important consideration for both wastewater and drinking water treatment is that the plastics are usually not destroyed, but rather transferred from one phase to another. Sludge disposal methods must therefore be considered because sludge application to land is a probable route for re-contamination of the environment. In many countries, sludge is disposed of in a landfill, incinerated to generate energy, or applied for nutrients in agriculture. Sludge must be treated to make it suitable for land application (Ahmad, Ahmad, and Alam 2016). Common sludge treatment methods include lime stabilization, composting, anaerobic digestion, and thermal drying. Despite this observation, sludge is still a significant source of MP post-treatment (Mahon et al. 2017). Both Mahon et al. (2017) and Zubris and Richards (2005) found more smaller MP in lime-stabilized sludge compared to anaerobically digested and thermally dried sludge, attributed possibly to higher pH and mechanical mixing. To date, there has not been much research into the removal of MP from water treatment sludge, though sludge is known to trap a large portion of the MP removed during water treatment and the return of sludge into the environment for agricultural purposes reintroduces MP back to the environment. Despite this, there are attempts to investigate alternative uses of wastewater treatment biosolids
Biosolids are retained at the WWTP for a relatively short time before being sent off site for land application and landfilling or incineration. Land application is the cost-effective option; it includes the spreading or tilling in on land or spraying of slurries on soils or plants/trees. Landfilling of the biosolids is typically used when land farming is not practical because this is more expensive than land application. To prevent biosolids from entering wetlands or waters of the United States, land appliers must ensure proper runoff control measures are in place. These protective measures include actions such as slope restrictions, buffer zones, tillage, dikes, and diversions (USEPA 1999). Sections 2.2.2, 2.3 and 2.4 provide more detailed information about the fate and transport of MP from biosolids.
Many WWTPs have difficulty finding land for biosolids application. Biosolids are one of the more difficult materials to bury in landfills. Biosolids landfilling options include disposal in a monofill (a landfill that accepts only wastewater treatment plant biosolids), or in a co-disposal landfill (a landfill that combines biosolids with municipal solid waste) (MSW). The biomass that is present on the leachate collection system of every modern landfill is trapping plastic particles and retaining them while the liner system is intact. It is highly unlikely that the anaerobic organisms found in today’s standard landfill will biologically process plastic in the timeframes of landfill life. When the landfill liners eventually fail, the retained plastic particles will be released into the resources beneath the landfill. An aerobic digesting landfill would likely process all incoming or created NP and MP available. It is uncertain if toxic components of the plastics would be released or consumed as the plastic particle is biologically reduced in an aerobic digestion landfill. Biological plastic processing is dependent upon particle size and acclimated microbes. Illustrations of chemical and mechanical processes associated with plastic particle size reduction, on their journey back to elemental components, are discussed by Meides et al. (2021).
Incineration of wastewater biosolids is the least common disposal method. Obviously, the plastic contained in the biosolids will be consumed and plastic will increase the British thermal unit (BTU or btu) value of materials being sent for incineration. The incineration feed can include all captured plastic materials that enter the WWTP, from NP to large plastic trash. Incineration is generally considered to be the ultimate eliminator for plastic waste, which can ultimately convert polymers into CO2 and mineral fraction (Geyer, Jambeck, and Lavender Law 2017). However, the unburned material from the bottom ash contained synthetic fibers (Chimenos et al. 1999), which implies that plastics and MP may still exist in the bottom ash and could be transported into the environment through its reuse or dumping. Yang et al. (2021) reported that MP were extracted from MSW incinerator bottom ash. The abundance of MP ranged from 1.9–565 number of particles per kilogram. The results suggested that incineration was not the ultimate eliminator of plastic waste, and bottom ash remained a potential source of MP.
There are also attempts to investigate alternative uses of water treatment biosolids, as summarized here. Rodríguez et al. (2010) probed the possibility of using spray-dried biosolids from DWTP as a cement additive and found addition of biosolids to impede cement hydration, hence its strength. Atomized biosolids also changed the setting behavior of the mixture and reduced mechanical strength of the standardized mortar even at 10% substitution, rendering it unsuitable as a cement additive. Nevertheless, a subsequent study by Dahhou et al. (2018) found DWTP biosolids could be incorporated into Portland cement, based on the mineralogical contents and durability. Cremades, Cusidó, and Arteaga (2018) revealed the potential use of biosolids from DWTP in the manufacturing of glazed tiles. Mixing clay with different percentages of spray-dried biosolids produced ceramic material that was environment-safe and passed the European leaching test and accelerated degassing tests.
Membranes. Tang et al. (2021) showed that membranes used in water filtration may be a source of MP, both because they are made of plastic materials such as polyamide (PA), polycarbonate (PC), and polytetrafluoroethylene (PTFE) and because they trap MP during filtration. However, in contrast, PTFE is practically insoluble in water and, therefore, is not mobile in the environment as a persistent, mobile, and toxic substance (Henry et al. 2018). The demand for membrane filtration has been foreseen to increase in the near future, leading to a market value of US$7.242 billion by 2025 (GlobalInfoResearch 2020). To meet the increasing demand, research into the potential of recycling disposed RO membranes and reusing them for nanofiltration and ultrafiltration has been launched.
Though the studies related to polymeric membranes generally focus on the reuse and recycling of the membranes and to a lesser extent on the synthesis of the membranes from discarded plastics, they contribute to the reduction of plastics input, hence MP in the environment. Equally, where membrane cleaning or back-flushing of filters is practiced, waste streams may be returned directly to the aquatic environment. Although use and disposal practices for waste products containing MP warrant special consideration, there are limited data available on the impact of such practices.
Filtration concentrate. There are very few studies looking into further treatment of the retentate or concentrate of membrane filtration to remove MP. Arola et al. (2019) highlighted electrodialysis or shear enhanced nanofiltration/RO concentrate for recovery of valuable components such as nutrients and struvite from the filtration waste streams of tertiary treatment in WWTPs but the study did not mention the elimination of MP from the waste streams. There are almost no studies on MP content of the reject streams of membrane-based water treatment plants. This presents challenges in understanding the severity of MP pollution caused by the reject streams. Mejía et al. (2017) showed the promising use of ultraviolet light and titanium dioxide in photo-oxidative removal of organic micropollutants such as diclofenac (>95%), ibuprofen (>95%), and naproxen (<65%) from the concentrate of a nanofiltration but, similar to Arola et al. (2019), the study is not MP-oriented.
Incineration. Waste incineration with energy recovery, as an essential part of the circular economy, accounts for a large proportion of solid waste treatment systems in both developed and developing countries. Incineration leads to the production of final bottom ash that is returned to the environment. To evaluate the efficiency of combustion, loss on ignition (LOI) is used as an indicator of the unburned material in the bottom ash. The LOI of bottom ash from two major furnace types, mass burn incineration and fluidized bed incineration, is 3%–5% and 2%–4%, respectively. Incineration is generally considered to be the ultimate eliminator of plastic waste, because it can ultimately convert polymers into CO2 and mineral fraction (Geyer, Jambeck, and Lavender Law 2017). However, there is some evidence that unburned material from the bottom ash contains synthetic fibers (Chimenos et al. 1999), which implies that plastics and MP may still exist in the bottom ash and could be transported into the environment through its reuse or dumping. This is backed up by a study examining bottom ash in 12 mass burn incinerators, one bottom ash disposal center, and four fluidized bed incinerators. Results indicated that although the abundance of MP differed significantly with local factors, bottom ash is a potential source of MP released into the environment (Yang et al. 2021).
6.2.2 Soil
Refer to Section 2.4 for a description of the environmental distribution, fate, and transport of MP in soil. The mitigation or remediation of MP as an emerging contaminant in soils has not yet been widely studied given the depth and breadth of the type and size of contaminants associated with MP.
MP have been detected in agricultural soils (Corradini et al. 2019, Dehghani, Moore, and Akhbarizadeh 2017, Liu, Adams, and Walker 2018, Ng et al. 2018, Panno et al. 2019, Piehl et al. 2018, Rillig, Ziersch, and Hempel 2017), particularly those to which biosolids compost has been applied (Corradini et al. 2019, Li et al. 2018, van den Berg et al. 2020); soils within suburban built environments (Liu, Adams, and Walker 2018); coastal soils (Zhou et al. 2018); forest soils adjacent to farmlands (Zhang and Liu 2018); and landfill refuse and leachates (He et al. 2019, Su et al. 2019, van Praagh, Hartman, and Brandmyr 2018). Apart from the aforementioned routes, MP enter soil ecosystems through agricultural mulching layers and wastewater irrigation (Conley et al. 2019, Kleinteich et al. 2018, Lares et al. 2018, Talvitie et al. 2017). Other pathways include landfills, beach litter, and runoff from farming and recreational, industrial, and urban spaces (Hurley and Nizzetto 2018). The remediation of other contaminants in soil has been widely applied for a variety of contaminants, such as volatile organic compounds, semi-volatile organic compounds, fuels, metals, and radionuclides.
The Federal Remediation Technologies Roundtable provides a list of 49 technologies that have been applied as a bench-scale pilot study or full-scale application to remediate as least one of the contaminant groups listed above (FRTR 2022). The table can help identify potential technologies that can be used to remediate MP in soil. There are two assumptions in this. First, the technologies applicable to semi-volatile organics, fuels, metals, radionuclides, and munitions may be applicable to MP. This is purely for screening technologies and not intended to imply that MP behave anything like the contaminants used to select the technologies. This leads to the second assumption: that remediation technologies for MP may not even exist yet or are on the cusp of development (for example, genetic engineered microorganisms to degrade plastics). As the market develops for mitigating MP contamination, so will the depth and breadth of the remediation technologies.
Several potential treatment technologies for use in removing MP from soil are listed in Table 6-4.
Table 6‑4. Potential treatment technologies for MP in soil
| Treatment Category | Treatment Technology | Media | Advantages/ Efficiencies | References |
| Biological | Developing Technology or Lab Scale | |||
| Biodegradation | Surface water, groundwater, wastewater, marine, soil, sediments |
75–99% A consortium of organisms can be used as a treatment strategy. MP removal efficiency |
Gan and Zhang (2019), Han et al. (2017), Hu et al. (2021), Pathak and Navneet (2017) | |
| Chemical | Developing Technology or Lab Scale | |||
| Electrochemical oxidation | Surface water, groundwater, marine, wastewater, soil |
58% MP removal efficiency, and up to 86.8% with an additional oxidant. Quick treatment time. Particularly effective for MP and NP destruction and effective for reducing MP size and mass and mineralizing NP. | Kiendrebeogo et al. (2021) |
|
| Physical | Developing Technology or Lab Scale | |||
| Thermal (that is, pyrolysis and gasification) | Surface water, soil | 54% in MP weight loss for catalytic advanced oxidation process with hydrothermal hydrolysis. |
Hu et al. (2021) |
|
| General Technology | ||||
| Incineration | Sludge/biosolids, soil, air | Can be used for energy generation. | Geyer, Jambeck, and Lavender Law 2017 |
|
6.2.3 Sediment
Refer to Section 2.5 for a description of the environmental distribution, fate, and transport of MP in sediment. The remediation of MP from sediments is still in the early stages of development and has largely been studied in the lab. Physical removal of nurdles and other MP has proven to be difficult due to the size, neutral buoyancy, and shape of MP and is largely limited to beach and river cleanups. Recent nurdle spills have prompted regulatory authorities to promulgate regulations and direct cleanup/removal activities. Publicly embraced volunteerism appears to be the most widely used effort to remove larger plastics and nurdles from shorelines, which can contribute to MP. Organized beach cleanups have encouraged the development of sifting machines, which require physically removing bucketfuls of sand. Only recently has identification of MP in sediments been successful and proven to be valuable for developing sampling strategies to locate pockets of plastic debris and their sources. Largely deposited by runoff, some MP have been traced back to litter, wastewater effluent, landfills, mining, industries, shipping, illegal dumping, the degradation and breakdown of larger plastic articles, land application of biosolids, and recreational activities and gear.
Biological degradation: The biodegradation of plastic in sediments in an aquatic environment differs greatly from other environments. Biodegradation can be complicated by adsorption of toxic chemicals and physical redistribution in the environment over time. Although various species of bacteria may be effective degraders, in an aquatic environment biodegradation may be incomplete, creating the need for additional measures.
Capping: Capping may be used to limit the migration of MP and would require effective management plans for wind, erosion, flooding, and precipitation. It may not be a long-term viable option for aqueous environments but may be an effective way to confine the movement of MP on a temporary basis.
Dredging: In order to landfill sediment with MP, first the sediment has to be dredged. MP does not necessarily have to be filtered/removed from the sediment. That would be needed only if the sediment was to be beneficially reused. If the sediment was to be landfilled, the MP does not need to be separated from the sediment. Physical separation of MP from sediment may be possible. Physical separation of dredge material size fractions has been performed on multiple sediment remediation projects—for example, Parsons (2019) and Tierra Solutions Inc. (2013). Separation of MP may require density separation in addition to size separation. Soil washing or mining ore processing methods may be applicable. The cost of separation may be prohibitive compared to landfilling the sediment (Parsons 2019, Tierra Solutions Inc. 2013)
Landfilling: Landfilling of sediments requires effective leachate containment/treatment, mitigation strategies, and effective monitoring plans for effluent, wind, erosion, flooding, and precipitation. Sampling and characterizing effluent should be enacted to eliminate the release of MP in leachate. The details of landfilling are provided in Section 6.1.4-Landfilling.
Physical separation/screening/washing: Physical separation, screening, and washing of sediments could be limited by depth, mesh size, access, and waste containment requirements.
6.2.4 Air
Refer to Section 2.6 for a description of the fate and transport of MP in air. Compared to the plethora of MP studies in the marine environment and the growing number of studies in terrestrial environments (Alimba and Faggio 2019, Auta, Emenike, and Fauziah 2017, Prata, Silva, et al. 2019), research on atmospheric MP has only recently gained attention. To date, very few studies have been conducted on atmospheric MP.
Filtration can be used to remove MP from air. Using a portable air cleaner or upgrading the air filter in your furnace or central heating, ventilation, and air-conditioning (HVAC) system can help to improve indoor air quality (USEPA 2018). Portable air cleaners, also known as air purifiers or air sanitizers, are designed to filter the air in a single room or area. Central furnace or HVAC filters are designed to filter air throughout a home. Typical filters are sized to remove particles of 10 µm, whereas high performance filters can remove particles as small as 0.2 µm. Portable air cleaners and HVAC filters can reduce indoor air pollution; however, they cannot remove all pollutants from the air. In addition, changing practices related to clothing drying could reduce or eliminate the quantity of MP fibers known to pass through dryer lint filters of mechanical air clothing dryers with external ventilation. Reducing textile washing frequency, improving lint removal by dryers (for example, improved lint screens or additional lint capture points), and modifying textile drying practices, such as by hanging textiles to dry or using nonvented condenser dryers, can reduce fiber release from textiles to air (Moran et al. 2021)