4 Human Health and Ecological Effects
A primary concern of MP pollution is whether they represent a risk to human health and ecosystems. This section presents information on (Figure 4-1):
- factors that influence the effects and toxicity of MP
- effects on human and ecological health due to exposures to MP in different environmental media and settings
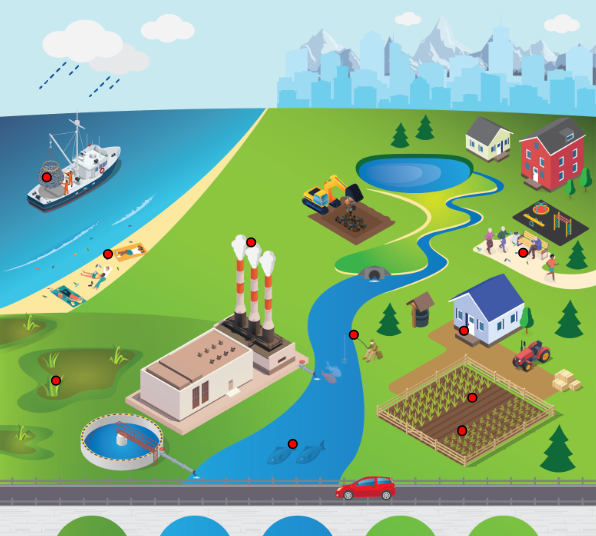
Figure 4-1. Conceptual model for human health and ecological effects.
Source: Jonathan McDonald and ITRC MP Team
Adverse effects on organisms that are exposed to MP can be separated into two categories: chemical effects and physical effects (Campanale et al. 2020). Understanding the effects of MP is complex due to different chemical and physical properties that make MP multifaceted stressors.
4.1 Chemical Properties
MP dispersed in the environment can contain substances that are not chemically bound to the polymer matrix. These substances may be derived from two sources: (1) additives and polymeric raw materials (for example, monomers or oligomers) originating from the plastics, and (2) chemicals absorbed from the surrounding environment (Campanale et al. 2020). These unreacted monomers, residual processing aids, and additives may be released during the plastic life cycle, potentially resulting in human and environmental exposure (Wiesinger, Wang, and Hellweg 2021).
Aging processes such as UV irradiation, biodegradation, physical abrasion, and chemical oxidation can affect the environmental behavior of MP. Different aging processes could affect the adsorption of pollutants, the leaching of additives, and the toxicity of MP (Luo et al. 2022). Luo et al. conducted a systematic analysis and summary of the environmental behavior and physicochemical properties of MP, as well as changes due to MP aging. The aging of MP affects their adsorption performance toward pollutants due to a series of changes in their specific surface area and oxygen-containing functional groups
4.1.1 Polymer Type and Additives
Microplastics are composed of a diverse suite of polymer types. All plastic polymers consist of repeating monomers, which form the backbone of the polymer. This backbone structure is the fundamental difference between polymer types, informing a plastic’s physical and chemical properties (Rochman et al. 2019). Several studies have suggested that the toxicity of MP depends on their polymer type, as well as on their size and shape (Rochman et al. 2019). It is important to distinguish between the toxic potential of plastic constituents and the potential for MP to release those constituents in the environment. Release of a constituent is based on the chemical properties of the polymer, the properties of the constituent, and the media into which it is being released (Teuten et al. 2009).
Different polymer types have different residual monomers that differ in toxicity; however, there is no reliable evidence that polymers themselves differ in toxicity. Some polymers, such as PVC and PUR, contain variable amounts of residual monomers that are carcinogenic or mutagenic at high concentrations, whereas the monomers from other polymers, such as PE and PP, are considered to have less significant hazard traits and endpoints (Rochman et al. 2019). Constituents of PVC can include carcinogens, such as diisononyl phthalate and di-2-ethyl-hexyl phthalate; a developmental toxicant, for example, diisodecyl phthalate; chlorides; stabilizing additives like tributyl tin, which cause endocrine disruption; and dioxins including 2,3,7,8-tetrachlorodibenzo-p-dioxin, which cause adverse impacts on immunity depending on the concentrations (Jaakkola and Knight 2008, Rochman et al. 2019, Thornton 2002). Styrene, the monomer for PS, is of concern because it is a classified carcinogen (Rochman 2015). The PC monomer, bisphenol A (BPA), is in the California Office of Environmental Health Hazard Assessment’s (OEHHA) Proposition 65 list of female reproductive toxicants and developmental toxicants (CA OEHHA 2020). Biomonitoring California also listed BPA as an endocrine toxicant (CECBP 2019). Although the PC monomer BPA may have endocrine disruption effects (Rochman 2015), the most recent FDA risk assessment concluded that BPA is safe at the current levels occurring in foods. The available information continues to support the safety of BPA for the currently approved uses in food containers and packing (FDA 2018).
Recent efforts have been taken to reduce the use of additives, especially phthalates, polybrominated diphenyl ethers, cadmium, lead, and BPA, in plastics. However, it is possible these substances are present in older plastics (WHO 2019). Low molecular weight and some mid-range phthalates such as di-2-ethyl-hexyl phthalate and dibutyl phthalate have demonstrated potential endocrine-disrupting effects. However, higher molecular weight phthalates have been assessed by USEPA and Health Canada and have no demonstrated potential endocrine disrupting effects
4.1.2 Adsorbed Chemicals
Hydrophobic interaction is the most common mechanism by which MP adsorb organic pollutants and control the portion of organic pollutants (Wang et al. 2018). MP can easily adsorb pollution in the environment because of their large specific surface area and strong hydrophobicity. Sorption is the process of transferring chemicals from fluids (liquids and gases) to solids (Fred-Ahmadu et al. 2020, Vieira et al. 2021) and includes adsorption and absorption. The sorption mechanisms between MP and chemicals depend on physical and chemical properties of MP, type of adsorbed chemicals, temperature, and solution chemistry (Wang et al. 2018). These parameters affect the amount of chemical adsorbed on MP, thus affecting the transfer of those chemicals in the environment and the food chain via MP (Wang et al. 2018).
MP present in surface water readily adsorb organic chemicals due to their hydrophobic nature, with the degree of hydrophobicity depending on their polymer type (Wang et al. 2018). Guan et al. (2022) showed in their studies that the aging time increased the oxygen content, specific surface area, and hydrophilicity of MP. The high surface area to volume ratio and hydrophobicity of MP contribute to the ability of MP to accumulate metals (for example, lead and cadmium) and persistent organic pollutants (POPs) such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), per- and polyfluoroalkyl substances (PFAS), and organochlorine pesticides such as dichlorodiphenyltrichloroethane (DDT) from marine water (Amelia et al. 2021, Scott et al. 2021).
MP may interact with environmentally persistent pollutants (for example, PAHs and metals) depending on the polymer physio-chemical properties (Teuten et al. 2009). Organic pollutants can adsorb to MP surfaces via hydrophobic, electrostatic, and non-covalent interactions (Joo et al. 2021, Lee, Shim, and Kwon 2014, Liu, Zhu, et al. 2019). Heavy metal adsorption to MP occurs through electrostatic interactions, van der Waals forces, and π–π interactions (Liu et al. 2021). Metals also interact with extracellular polymeric substances (metabolites secreted by microorganisms) from assemblages of microorganisms existing in biofilms that develop on MP surfaces (also discussed herein). The extent of metal adsorption to extracellular polymeric substances is a function of composition, functional group composition, and surface microstructure such as porosity or surface roughness (Liu et al. 2021).
The sorption and aggregation that occur between weathered MP and their co-existing constituents is influenced by the weathering rate of MP in the aquatic environment. The weathering process increases the oxygen-containing functional groups and the specific surface area of MP. More studies are needed to investigate the weathering processes of diverse MP under natural field conditions in soil, sediments, and aquatic environments to better understand the impact of weathered MP in the environment (Duan et al. 2021).
Degradation of the surface of MP and weakening of the plastic surface cause the release of microscopic particles and increase accessibility to microorganisms (Lambert, Scherer, and Wagner 2017). The increased capacity to adsorb harmful chemicals in smaller MP is attributed to higher surface area to volume ratios (Bergmann, Gutow, and Klages 2015, Rochman et al. 2019, Wang and Wang 2018a). Degradation of MP also enhances the sorption capacity to harmful chemicals due to increased surface area (Alimi et al. 2018). Compared to smooth MP, rough MP adsorb more chemicals such as DDT due to increased surface area from degradation. This results in increased sorption capacity (Alimi et al. 2018). These contaminants have a greater affinity for plastics than water; hence, the concentrations on MP are orders of magnitude greater than in the ambient water (Cole et al. 2011, Smith et al. 2018).
Color additives (that is, pigments), aging, UV weathering, discoloration, fouling, and polymer type may also affect chemical sorption capacity of MP (Alimi et al. 2018, Endo et al. 2005, Teuten et al. 2009, Wang et al. 2018). Discoloration of MP occurs with weathering and aging processes. Aged MP have higher sorption capacities of pollutants than do virgin ones (Guo and Wang 2019). Pigments contain organic chemicals from plants and animals that may enhance chemical sorption capacity of colored MP (Wang et al. 2018). Antunes et al. (2013) showed that aged and black MP pellets contained higher concentrations of PCBs and PAHs than colored and white ones. In addition, aged pellets contained higher DDT concentrations than others (Antunes et al. 2013). All pellets contained mainly PE and PP, but some black pellets also contained PUR, which is known to adsorb more contaminants than other polymer types due to its higher surface area (Antunes et al. 2013). Aged MP have longer exposure times and higher degradation rates than non-aged ones. The increased surface area and roughness result in increased chemical sorption capacity (Antunes et al. 2013, Wang et al. 2018)
4.2 Physical Properties
Polymer type affects the physical properties of MP, such as density, crystallinity, hydrophobicity, and porosity (Lambert, Scherer, and Wagner 2017, Wang et al. 2018). Crystallinity is the degree of structural order of polymer chains that make up the MP. Crystallinity affects the density and permeability of MP. The crystallinity of MP can change via degradation. Degradation in the amorphous region of MP increases its overall crystallinity and consequently influences other physical properties, such as surface area, shape, size, and density. Thus, the toxicity of degraded MP may differ from the parent MP (Lambert, Scherer, and Wagner 2017). Pore diameter and size of the additive and the pore size of the polymer determine the migration potential of an additive in a polymer. Smaller or lower molecular weight additives move more easily through a polymer that has a bigger pore size (Teuten et al. 2009).
The toxicity of MP is influenced by size (Bucci and Rochman 2022, Lambert, Scherer, and Wagner 2017). The toxicity of small MP is a particular concern because they can transfer between tissues and cells of organisms (Rochman et al. 2019). MP smaller than 10 µm can be inhaled by humans, and MP larger than 1 µm can be removed from the respiratory tract without reaching the lungs (Wright and Kelly 2017). MP less than 1 µm may translocate via diffusion and penetrate into lung tissues (Wright and Kelly 2017). A fish study showed that MP particles smaller than 130 µm may translocate between cells through diffusion (McIlwraith et al. 2021). Exposure to smaller MP that can penetrate through lipid membranes of cells causes the formation of a higher number of reactive oxygen species, which result in growth inhibition and behavior alteration in various aquatic organisms and mammals, such as rats and humans, through oxidative stress and inflammatory responses (Hu and Palić 2020). However, further mammalian model and epidemiological studies are needed to confirm these findings (Hu and Palić 2020). To date, these studies are limited, and laboratory conditions often do not reflect real-life environmental conditions. Although there are limited studies that showed translocation of MP, more research is needed in this area.
The shapes of MP affect the interactions within the biological systems (Lambert, Scherer, and Wagner 2017). MP particles with more irregular shapes or fibers may attach more readily to internal and external surfaces of organisms (Lambert, Scherer, and Wagner 2017). Many nonfiber MP are likely to sink due to their higher density than fibers (Miller, Hamann, and Kroon 2020). Therefore, bivalves that filter the water column are more likely to be exposed to fibers absorbed to the water column than to other shapes of MP that sink (Miller, Hamann, and Kroon 2020). In a recent study, ingestion of PE microfibers (700 µm in length, 10–15 µm in diameter) resulted in more oxygen consumption in juvenile Centropristis striata (black sea bass) than ingestion of PE microspheres (10–20 µm), which suggests microfibers cause more risk to the respiratory system in aquatic organisms than MP spheres (Stienbarger et al. 2021). In another recent study, 38% of the fish collected from San Francisco Bay, California, contained MP levels above laboratory blanks; the average number of MP ranged from 0.2 to 0.9 nonfiber MP per fish and 0.6 to 4.5 fiber MP per fish (Sutton et al. 2019). In this study, a total of 1,919 MP particles were counted in 152 fish gut samples. The distribution of MP in all fish gut samples was 1,650 fibers (86%), 192 fragments (10%), 74 films (3.9%), two foams (0.1%), and one sphere (0.05%) particle (Sutton et al. 2019). MP with spherical shapes were shown to cause less injury and gut inflammatory reaction than irregular shapes (Pirsaheb, Hossini, and Makhdoumi 2020). For instance, PP fibers, with a length of 20–75 µm and diameter of 20 µm, have a higher toxicity to the Hyalella azteca (an amphipod) than PP beads with a diameter of either 10 or 27 µm (Lambert, Scherer, and Wagner 2017). Sharp-edged and rough MP can cause more mechanical injuries to the gut epithelium in organisms than smooth ones (Kutralam-Muniasamy et al. 2020b)
4.3 Microplastics as Vectors
Concerns have been raised that unreacted residual monomers, chemical additives (for example, plasticizers, flame retardants, antioxidants, and pigments), processing aids, by-products, breakdown products, and contaminants present in plastics can be harmful to the environment and human health when they leach from the plastic polymer matrix (Smith et al. 2018, Wiesinger, Wang, and Hellweg 2021, Wright and Kelly 2017). The photolytic or mechanical degradation of macroplastics into MP may cause additives to leach out, thus introducing potentially harmful chemicals to biota (Cole et al. 2011). Plasticizers, other plastics additives, and constitutional monomers can leach from MP in waste disposal sites into groundwater and surface waters; thus, MP can serve as carriers of organic contaminants to wildlife (Teuten et al. 2009). For instance, BPA, phthalates, and alkylphenols such as nonylphenol and octylphenol in landfill leachate have been detected that can end up in the aquatic environment (Teuten et al. 2009). In addition, MP can serve as a substrate for microbial communities and can carry pathogenic and invasive species to non-native waters (Cole et al. 2011). Metal-based additives in plastics are mainly used as inert fillers, pigments for color, and stabilizers (Turner and Filella 2021).
Ingested MP have also been hypothesized as a vector for the transport of certain persistent pollutants. For instance, ingestion of MP fibers by the crab Carcinus maenas and lobster of the genus Nephrops formed fibrous aggregation within the gut and reduced egestion times. MP are more likely to transfer to their predators with increased residence time (Au et al. 2017). Shape and size influence MP residence time in prey species (Au et al. 2017, Gray and Weinstein 2017). Smaller MP can have longer gut retention and may be transferred from lower to higher trophic organisms through the food web (Gray and Weinstein 2017).
Kutralam-Muniasamy et al. (2020a) showed that organisms consume more aged MP than virgin MP. Moreover, many animal species are visual predators, and may select prey based on the color of MP (Carlin et al. 2020). Therefore, some animal species may selectively feed on MP that contain larger amounts of toxic chemicals (Carlin et al. 2020, Guo and Wang 2019, Kutralam-Muniasamy et al. 2020a).
PET is one of the most common of MP. Fiber and fragments resulting from degradation of larger items, such as synthetic clothing and fabrics, would release microfibers when worn and washed. PET is also widely used in packaging materials, beverage bottles, and functional material. The amount of PET that enters and accumulates in the ecosystem poses a significant environmental challenge (Gong et al. 2018). PET is suspected to leach endocrine-disrupting chemicals, depending on the product type (Lambert, Scherer, and Wagner 2017, Sax 2010).
A recent review of 61 studies evaluated the weight of evidence for the MP vector effect as generally weak (Koelmans, Diepens, and Mohamed Nor 2022). Laboratory and field studies often neglected environmentally relevant exposure conditions or were inconclusive with respect to causality (that is, did not address alternative yet reasonably complete exposure pathways). The more conclusive laboratory and field studies generally provided evidence for the absence of the vector effect. Modeling studies under environmentally realistic conditions suggest that MP may act as passive samplers (or sinks) in the gut that counteract the bioaccumulation mechanism. Thus, the available evidence does not support the assertion that MP play a major role in the bioaccumulation of POPs when compared to other exposure pathways under environmentally realistic conditions. However, concentrations of MP and associated chemicals ultimately determine the extent to which the presence of MP increases chemical exposure compared to a contaminated environment without plastic particles (Koelmans, Diepens, and Mohamed Nor 2022).
The large surface area to volume ratio and hydrophobic nature of MP also provide a suitable microenvironment for the formation of biofilms on the particle surface (Katyal, Kong, and Villanueva 2020). Biofilms consist of single or multiple species of microorganisms attached to a solid surface and encased in an extracellular polysaccharide matrix or extracellular polymeric substances (Bowley et al. 2021). Certain MP biofilms have been shown to exhibit selective enrichment of bacterial pathogens, including antibiotic-resistant bacteria (Galafassi et al. 2021, Laverty et al. 2020, Oberbeckmann, Löder, and Labrenz 2015, Oberbeckmann et al. 2014, Sathicq et al. 2021, Yang et al. 2020). Although MP can serve as a vector for pathogens, it remains uncertain whether the increasing number of MP in aquatic habitats facilitates pathogen transmittal and increases the potential for disease outbreaks (Bowley et al. 2021)
4.4 Trophic Transfer
Trophic transfer is a process of indirect exposure wherein humans or wildlife predators consume an organism that has retained MP. MP have been shown to occur in 800 species encompassing all trophic levels (primary consumers, herbivores, predators) and habitats—for example, in marine coastal seagrass, reefs, and open water (Akhbarizadeh et al. 2020, BfR 2020, Chatterjee and Sharma 2019, EFSA Panel on Contaminants in the Food Chain 2016, GESAMP 2016, Li et al. 2019, Sharma et al. 2021, Mercogliano et al. 2020, Smith et al. 2018, GESAMP 2015). Food webs consist of a network of trophic interactions that could elucidate ecosystem processes and functions. However, the presence of unknown but critical networks hampers the understanding of complex and dynamic food webs in nature. Kuwae et al. (2012) demonstrated that direct predator-prey relationships between shorebirds and biofilm are mediated by multiple ecological and evolutionary determinants. A major impediment in determining food web structure stems from the difficulty in identifying interspecific links. In general, the discovery of new interactions in networks derives from extensive empirical studies (Bascompte 2010). Kuwae et al. (2012) empirically showed that a missing and critical trophic link does exist by exposing extensive predator-prey relationships between shorebirds (waders) and biofilm. The strength of this missing link is differentially mediated by predator species traits, the environment that determines node properties (food density) and evolutionary history (phylogenetic constraints).
Lower trophic–level filter feeders ingest MP particles that are mistaken for food due to their similar characteristics, such as size, color, buoyancy, and density (Akhbarizadeh et al. 2020, Chatterjee and Sharma 2019, Lusher, Hollman, and Mendoza-Hill 2017, Sharma et al. 2021). These include plankton (Laist 1987), zooplankton (Cole et al. 2013, Lee et al. 2013, Lin 2016), amphipods (Au et al. 2015, Straub, Hirsch, and Burkhardt-Holm 2017), bivalves (Farrell and Nelson 2013, Van Cauwenberghe and Janssen 2014, Wright, Thompson, and Galloway 2013), marine worm (Besseling et al. 2013), crab (Brennecke et al. 2015, Farrell and Nelson 2013), and planktivorous or larval fish (Besseling et al. 2013, Critchell and Hoogenboom 2018, Lu et al. 2016, Pedà et al. 2016, Steer et al. 2017). Additional work shows that some larval fish ingest the majority of plastic via trophic transfer (Athey et al. 2020, Hasegawa and Nakaoka 2021, Stienbarger et al. 2021). MP content in upper trophic–level vertebrates (predatory fish, sea birds, and mammals) demonstrates trophic transfer because they are unlikely to confuse their prey and plastic (Azzarello and VanVleet 1987, Mattsson, Hansson, and Cedervall 2015, Sharma et al. 2021). Trophic transfer of MP can also occur when prey has MP externally adsorbed to appendages (Cole et al. 2013, Gutow et al. 2015, Setälä et al. 2018).
There have been concerns about biomagnification or concentrating MP in higher trophic–level organisms as a result of ingesting other plants or animals. However, quantitative studies showing biomagnification of MP and the health implications for humans (Derraik 2002, McIlwraith et al. 2021, Moore 2008, Setälä et al. 2018, Teuten et al. 2009) do not indicate clear evidence of biomagnification (Gouin 2020).
Bioaccumulation is the net accumulation of a chemical by an organism resulting from uptake via all routes of exposure (sediment, water, and food). Traditional assessments use the information from bioaccumulation laboratory studies to generate a bioconcentration factor (BCF), which is the ratio of the steady-state chemical concentration in an aquatic organism and the water. For MP, bioaccumulation would represent accumulation of MP within the tissues of an organism that exceeds the concentration of such particles in the surrounding environment.
Mackay and Fraser (2000) published a review that presented the bioaccumulation of organic substances in organisms, especially fish. Based on a review of existing estimation methods, a tiered predictive approach was developed for addressing the large number of chemicals of commerce. The simplest Tier 1 approach is an empirical correlation for BCF as a function of the octanol–water partition coefficient. The Tier 2 evaluation predicts the bioaccumulation factor by using a mechanistic mass balance model applied to the organism at steady state in which relevant uptake and loss processes are quantified. The equivalence of rate constant and fugacity models is demonstrated and methods of obtaining parameter values are discussed. Such a model reveals the relative significance of gill ventilation, food uptake, egestion, and metabolism. The most detailed Tier 3 evaluation involves prediction of the potential for biomagnification in a food chain involving both fish and air-breathing animals (Mackay and Fraser 2000).
However, due to analytical and methodological issues, there have been very few, if any, laboratory studies conducted on the bioaccumulation of MP particles in higher organisms such as fish. To more fully address the issue of bioaccumulation and trophic transfer, Gouin (2020) reviewed over 800 publications reporting the presence of MP in fish. The review included data for >900 species representing ~ 87,000 individual organisms. MP (and macroplastic debris) were observed in about 17,500 or 20%. On average, there were 4 particles reported per individual organism across all studies reviewed.
Although fish muscle is the most frequently consumed fish tissue for humans, other tissues (gill, liver, gut) are more frequently analyzed because these are more likely repositories for contaminants (Akhbarizadeh, Moore, and Keshavarzi 2018). Fish and seafood, particularly those consumed whole (for example, canned sardines), represent some of the most important routes of MP exposure for humans through the diet (Akhbarizadeh, Moore, and Keshavarzi 2018, GESAMP 2015, 2016, Karami et al. 2018, Lusher, Hollman, and Mendoza-Hill 2017, Mercogliano et al. 2020, Smith et al. 2018, Yang, Yang, et al. 2015).
Physical properties of MP (for example, size and shape) can influence not only uptake, but also retention duration in biota (Ašmonaitė and Almroth 2019). More research is needed to understand the biological fate of plastic particles in an organism following uptake in the digestive tract—translocation (to the circulatory system or surrounding tissue), accumulation (retention), and elimination (egestion) (Browne et al. 2008, Lusher, Hollman, and Mendoza-Hill 2017). Most investigations pertain to the presence of MP in the gut tissue of human food items. Elimination is the dominant fate pathway; likely greater than 90% of MP accumulated in humans are excreted in feces (Liebmann et al. 2018, Schwabl et al. 2019, Smith et al. 2018).
In addition to trophic transfer of the MP particles, there may be other co-occurring contaminants that could also transfer (Mercogliano et al. 2020). Laboratory data demonstrated that trophic transfer of POPs associated with MP does occur from brine shrimp (Artemia nauplii) to zebrafish (Danio rerio) (Athey et al. 2020, Batel et al. 2016, Hasegawa and Nakaoka 2021). According to Athey et al. (2020), Batel et al. (2016), Hasegawa and Nakaoka (2021), Lusher, Hollman, and Mendoza-Hill (2017) these contaminants would represent less than 0.1% of the total dietary intake.
Trophic transfer across terrestrial systems has not been well studied. Studies have shown the impact of MP and chemicals associated with MP on plants; however, there is uncertainty as to whether plants take up MP into the edible parts. A study by (Wang et al. 2022) showed that phthalate acid esters associated with plastics and MP were absorbed by roots and transferred to leaves. This data gap on trophic transfer of MP in terrestrial systems should be explored to further understand transport pathways of MP into food sources for both humans and wildlife (Figure 4-2).
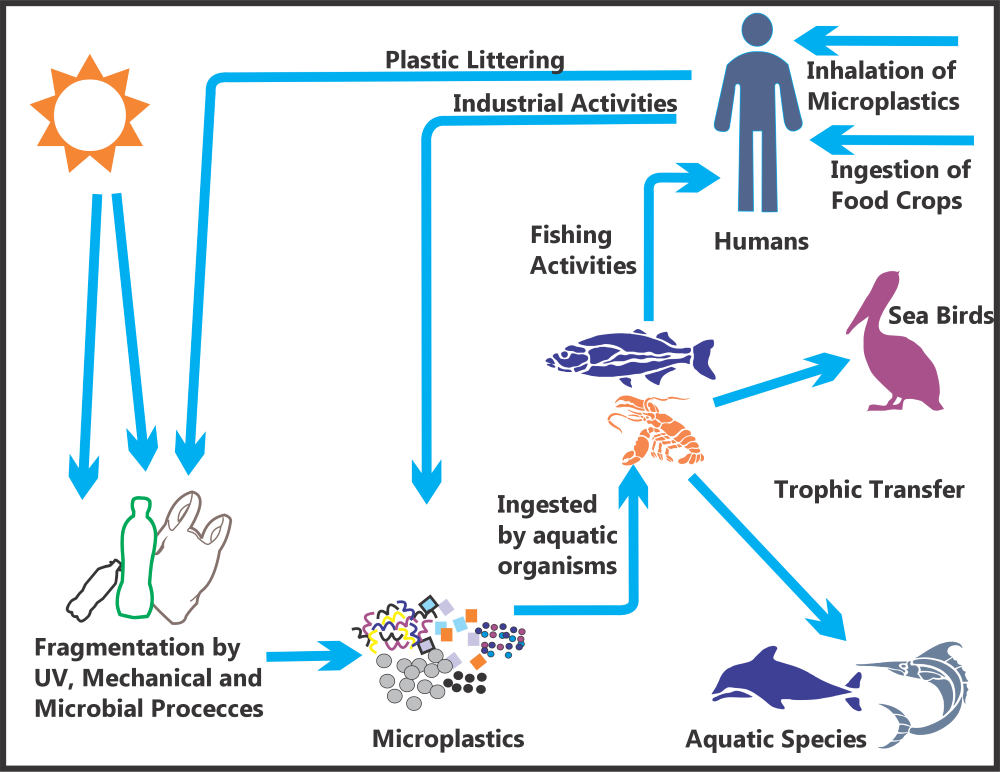
Figure 4-2. MP from primary and secondary sources enter the food chain at all trophic levels.
Source: Modified from Issac and Kandasubramanian (2021)
4.5 Human Health
As with all contaminants, the type and extent of MP toxicity will be directly influenced by the degree of exposure (dose and duration) from the pathways and routes described. As discussed in Section 4.1, MP characteristics such as size, density, composition, hydrophobicity, and surface charge influence the uptake, absorption, and distribution of MP within the human body, thereby directly influencing potential adverse health exposures and effects. Potential exposures and impacts of MP to human health are increasingly being considered by expert panels, for example USEPA (2015), European Commission (2019) and NASEM (2021). As described in the following sections, other panels and regulatory bodies have focused on food as a source of exposure.
4.5.1 Potential Human Exposures
Humans are known to be exposed to MP from a variety of sources, with a growing number of attempts to quantify intake. Humans can be exposed through ingestion, inhalation, and dermal absorption, with primary exposures through inhalation of airborne particles and ingestion of contaminated food (Mohamed Nor et al. 2021). There is less evidence of systemic exposure through skin contact. Mohamed Nor et al. (2021) developed a probabilistic model to estimate the global MP intake per capita per day through inhalation and ingestion of each of eight foods (fish, mollusks, crustaceans, tap water, bottled water, beer, milk, and salt) for which MP concentrations were available. The MP intake distribution was very broad. Median intake for inhalation and all eight foods was 1.8 x 10-4 and 5.8 x 10-4 mg per capita per day for children and adults, respectively. Mohamed Nor et al. (2021) noted that this is far less than the dietary intakes of other types of nano- and microparticles, such as titanium dioxide and silicates, that were estimated by Powell et al. (2010) to be about 40 mg/capita/day in the United Kingdom. Mohamed Nor et al. (2021) also simulated transfer of a relevant suite of chemicals from ingested MP via kinetic modeling. The results showed that the contribution of ingested MP to total chemical intake would not substantially affect the background chemical concentration in the gut originating from food. Other studies attempting to provide an overview of MP exposures from multiple sources have focused on dietary exposures (Jin et al. 2021, Pironti et al. 2021) or MP output in feces (Braun et al. 2021, Yan et al. 2022).
Another line of research includes studies that have reported on the presence of MP in various tissues in humans, including placenta (Ragusa et al. 2021), liver (Horvatits et al. 2022), and breast milk (Ragusa et al. 2022). Pulmonary translocation to systemic circulation has been demonstrated for fine and ultrafine particles (Elder and Oberdörster 2006, Peters et al. 2006).
4.5.1.1 Food and Water
Humans can be potentially exposed to MP through ingestion of contaminated food and drink items (Jin et al. 2021, Thornton Hampton et al. 2022). Literature reviews on the occurrence of MP in food can be found in reports issued in 2019 by the Science Advice for Policy by European Academies consortium (SAPEA 2019) and the Norwegian Scientific Committee for Food and Environment (Skåre et al. 2019). Notably, Skåre et al. (2019) concluded that there were very limited data of acceptable quality on levels of NP and MP in foods, and that an exposure assessment for human exposure to NP and MP could not be done. The European Food Safety Authority, EFSA Panel on Contaminants in the Food Chain (2016) presented results from single studies on the presence of MP (fibers and fragments) in honey, sugar, and beer, but the origin of the MP in these foods was not identified.
Food packaging is an important source of exposure due to the extensive use of plastic as the preferred packaging material worldwide. Plastic packaging materials and containers are commonly made up of thermoplastic resins, specifically PET, high-density polyethylene (HDPE), low-density polyethylene (LDPE), polypropylene (PP), polyvinyl chloride (PVC), and polystyrene (PS). The heavy use of plastic food containers, plastic packaging, plastic bottles, disposable cups, infant feeders, plastic-coated metal, and paper cartons has provoked the issue with direct contact and release of plastic flaking into food items. People store, transport, prepare, and consume food in plastic containers without an awareness of the potential leaching of plastic from containers into their foods and drinks (Jadhav et al. 2021). EFSA concluded that available toxicity and toxicokinetic data for both MP and NP were insufficient to support human risk assessment for these constituents in foods. Areas of needed research on occurrence were identified (EFSA Panel on Contaminants in the Food Chain 2016). The following paragraphs summarize reviews with the most available data on MP concentrations in foods. These include new studies completed after the EFSA report was issued: seafood, salt, and drinking water (tap water and bottled water).
4.5.1.2 Soil and Indoor Dust
Exposure to MP in residential soil and indoor dust could occur through incidental ingestion, inhalation of resuspended particles, and dermal contact. Very few studies have examined the presence of MP in soil, and there are no quantitative estimates of the magnitude of exposure to MP in soil. MP concentrations in residential soil are expected to be very low unless affected by a localized source (such as MP shed from plastic structures or furniture in a yard). To date, most studies on MP in soil consider MP introduced into agricultural soils by soil amendments and consider uptake into crops and produce (de Souza Machado, Kloas, et al. 2018, Guo et al. 2020) or potential leaching to groundwater (Wanner 2021) rather than direct contact by humans. Based on the low exposure potential demonstrated in these studies, further investigation of direct human exposure to MP in soil is considered a low priority.
In contrast with soil, indoor dust has been shown to contain high concentrations of MP (largely from clothing fibers) with possibly as much as 30% of household dust consisting of MP (Dris et al. 2017). Household dust is thought to be the primary route of inhalation exposure to MP (Zhang, Xu, et al. 2020, Zhang, Zhao, et al. 2020). Incidental ingestion of MP from indoor dust may also occur via hand-to-mouth activity and by swallowing some fraction of inhaled MP. Inhaled particles can become trapped in the upper airways and swallowed (Wright et al. 2021), or they can be directly transported into the lung tissue (van Dijk et al. 2021).
Both indoor and outdoor ambient air pose a unique risk to human health as atmospheric fallout (particles deposited by air currents) and household dust loaded with MP from carpets, furniture, clothing, and building materials (paints, insulation, and countertops) are commonplace (Chen, Feng, and Wang 2020, Enyoh et al. 2019, Mbachu et al. 2020, Wright et al. 2021, Zhang, Wang, and Kannan 2020). Vianello et al. (2019) used a breathing manikin to investigate ambient indoor air in three apartments. All samples were contaminated with MP at concentrations of 1.7–16.2 particles/m3. In addition, all three blank samples showed the presence of MP at 7.7 ± 3.8/m3, indicating cross-contamination. On average the number of MP inhaled by the manikin over 24 hours was 9.3 ± 5.8/m3
4.5.1.3 Household Water and Personal Products
Dermal contact with MP can occur through contact with household water during activities such as handwashing, bathing, or showering in water that contains MP and using MP-containing products like hand cleansers, face washes, face masks, and toothpaste (Abbasi et al. 2019, Morgan and DeLouise 2020). MP released while showering can also be inhaled, and MP in toothpaste may be ingested.
Focusing on dermal exposures, the German Federal Institute of Risk Assessment has determined that the MP particles used in exfoliants and shower gels are much larger than 1 µm (BfR 2019) and unlikely to be absorbed systemically. Because uptake of particles across the skin would entail the penetration of the stratum corneum, which is limited to particles below 100 nm, absorption of MP through the skin is thought to be unlikely (Yee et al. 2021). Thus, it may be more likely that NP (<100 nm) could traverse the dermal barrier (Prata, da Costa, et al. 2020). Additionally, uptake of MP through the skin is thought to be more likely if an individual has a barrier defect in their epidermal layer, such as those that occur due to UV radiation exposure (Morgan and DeLouise 2020)
4.5.1.4 Outdoor and Indoor Air
Few studies have examined MP concentrations in outdoor or indoor air, but these sources are receiving increasing attention. A study from China suggests MP may be widely present in urban air (Zhu, Huang, et al. 2021). Concentrations in northern cities (358 ± 132 items/m3) were higher than those in southeast cities (230 ± 94 items/m3). Most airborne MP were smaller than 100 μm, and most particles were fragments. PE, P, and PS were the dominant polymers. High concentrations in indoor air were suggested by a study of MP fallout in indoor environments (Zhang, Zhao, et al. 2020), which found the highest average MP fallout was 9.9 × 103 MP/m2/d in a dormitory, 1.8 × 103 MP/m2/d in an office, and 1.5 × 103 MP/m2/d in a corridor.
Researchers have studied a variety of contaminants found in artificial turf and different types of infill used to soften its surfaces. In a Collaborative Health and the Environment (CHE) webinar, Rachel Massey, Lindsey Pollard, Zhenyu Tian, and Sarah Evans discussed their work at the Toxics Research Institute looking at environmental health impacts of artificial turf and safer alternatives (Massey et al. 2022). Most commonly, synthetic turf is built by layering various kinds of plastics with polyurethane adhesives on top of a bed of gravel (which provides drainage). Like all plastic materials, synthetic turf reportedly breaks up and sheds massive amounts of tiny plastic particles into the environment and our bodies. A 2018 report (ICF 2018) for the European Commission showed that athletic fields composed of synthetic turf shed an annual average of 18,000–70,000 tons of MP each year into surrounding air, soils, and waters. Infill material is added to absorb impacts to help prevent injury and to mimic the feel of natural turf. This material is usually polymeric and in the form of small particles. Artificial turf for domestic applications is unlikely to contain plastic infill material as it is both costly and unnecessary for this purpose. The pile fibers may wear or break and form MP, but this is expected to be minimal compared with sports turf that is subject to a great deal more abrasion. The potential magnitude of MP exposure via inhalation has also been considered in several studies (Cox et al. 2019, Prata, da Costa, et al. 2020).
MP inhalation risks can be exacerbated in the occupational setting where workers must perform their job functions in potentially high concentrations of airborne MP particles (Murashov et al. 2021). Industrial settings where high MP ambient air loads are present include plastic manufacturing, especially 3D printing (Stefaniak et al. 2017, Stephens et al. 2013), and in the nylon flock industry (Zarus et al. 2021).
A recent study by Jenner et al. (2022) detected MP in all regions of the human lung using FTIR. MP were found in 11 of the 13 lung tissues evaluated. PP and PET were the most common polymers found in the lung, with fibers and fragments being the most common shapes. MP were significantly more abundant in the lower lung than both the upper and middle lung. This was the first study of its kind and supports that inhalation is a route of exposure for humans to environmental MP.
4.5.2 Potential Health Effects
To date, there is limited research on the potential health effects of MP on human health, but new studies are being published at a rapidly accelerating pace (Figure 1-5). Studies on health effects directly in humans are mainly limited to occupational studies of inhalation exposures. Most research has relied on extrapolation of results from animal bioassay (rodent, fish) model systems using PET, PS, or PE, or on in vitro assays using human cell lines. Studies are most often conducted on virgin polymer spheres rather than on weathered polymers or MP with adsorbed environmental contaminants that humans are most likely to be exposed to. Further studies in translatable model systems are needed to predict the impacts of human exposure to MP.
A key data gap in understanding the impacts of MP in humans is using toxicity data modeled from animal bioassays. To date, researchers have identified a lack of mammalian or cell exposure studies that show the toxic effects due to MP exposure. Animal bioassays suggest that there could be potential concerns for respiratory toxicity, immune effects, and digestive and excretory toxicity in humans. However, results from animal bioassays have not shown any pathological effects following exposure to MP. Key endpoints following ingestion and inhalation exposure are discussed further below, with a preference for data obtained from animal bioassays. Dermal contact has not been identified as an exposure route of concern for MP and is therefore not discussed.
Once taken into the body, MP may cause adverse health effects through at least three different processes:
- adverse effects to target organ(s) via the physical presence of the MP particle (Blackburn and Green 2021).
- adverse effects due to toxicity via infection or disruption of microorganisms on the MP gut microbiota (Tamargo et al. 2022).
- direct toxic effects caused by the chemical components within the MP. Co-exposure of MP and other chemicals has been identified as a potential concern.
Some studies have also suggested that MP may possibly add to or synergize the adverse effects of the chemicals that they contain or have absorbed (Bhagat, Nishimura, and Shimada 2021, Kaur et al. 2022).
4.5.2.1 Health Effects Due to Ingestion
New reviews of ingestion-related health effects for MP are continually being published and focus on both the nature and quality of the available data (Coffin 2022, Vethaak and Legler 2021). Health effects reported in various mammalian ingestion studies are for PS spheres less than 20 μm, Biomarkers of inflammation and oxidative stress were health effects that were reported more consistently than reproductive and tissue-level responses (Coffin 2022).
Following oral ingestion, it is hypothesized that MP smaller than 20 μm (Campanale et al. 2020) may be directly absorbed (Ensign, Cone, and Hanes 2012, Powell, Thoree, and Pele 2007, Yong, Valiyaveettil, and Tang 2020) or can enter the gut mucosa via engulfment from cells within the intestinal lymphoid tissue. Similar processes have been demonstrated in the rat and in mouse models wherein ingested MP were then identified in the gut, liver, spleen, and/or kidney (Campanale et al. 2020, Deng et al. 2017, Prata, da Costa, et al. 2020, Rahman et al. 2021, Yong, Valiyaveettil, and Tang 2020). However, there have been concerns regarding some of these data due to mass imbalance and incongruities within the reported data (Braeuning 2019, Stock et al. 2019). As stated above, the database is extremely limited, and some oral exposure studies in rodents have been negative (that is, no treatment-related adverse effects were seen on organ weights or histopathology), or only exhibited positive results with concentrations of MP whose environmental relevance is unknown. Similar to inhalation, effects seen in mice following ingestion of high concentrations of PS MP are largely immune responses, including a reduction in mucus secretion, gut barrier dysfunction, intestinal inflammation, and gut microbiota dysbiosis.
One of the few available rodent oral bioassays dosed mice with either 5 or 20 μm PS particles via gavage at low (0.01 mg/day), medium (0.1 mg/day), or high (0.5 mg/day) dose levels for four weeks (Deng et al. 2017). Histological evaluation of the liver was conducted on control and high dose animals only and biochemical analysis was conducted on serum of all dose groups. MP particles of both sizes were found in the liver, kidney, and gut of the exposed mice. Effects observed included significantly decreased liver weights in the high dose mice, and increased inflammation and lipid droplets. Decreased adenosine triphosphate (ATP) levels and increased lactate dehydrogenase activity in exposed mice were dose dependent. The authors also reported indications of oxidative stress, changes in metabolomic profiles, increased activity of acetylcholinesterase (AChE), and changes in serum neurotransmitters in the exposed mice (Deng et al. 2017). The reader is referred to the primary study (Deng et al. 2017) and subsequent reviews (Coffin et al. 2022) for more detailed descriptions of dose and effects estimates. A neurobehavioral study by Rafiee et al. (2018) on rats fed PS NP (reported to have an average diameter of ~40 nm) for 5 weeks did not detect any significant behavioral changes. Although both studies are controlled mammalian laboratory studies, some have argued that the findings are of questionable human relevance due to the high doses, short durations, and unclear clinical relevance. See WHO (2019) and Yong, Valiyaveettil, and Tang (2020) for critical reviews of these studies.
Studies focused on the immunological responses in fish and other aquatic organisms as well as certain mammals (for example, mice) have found that exposure to MP affects their immune systems (Hirt and Body-Malapel 2020). Immune system effects are influenced by the chemical composition of the material and physical factors, including the particle size and shape (Morgan and DeLouise 2020). Factors like the presence of monomers and endogenous additives and their ability to migrate may also play influential roles.
Additionally, environmental exposure to MP in genetically susceptible individuals may disrupt their immune function, which could lead to autoimmune diseases or immunosuppression (Rahman et al. 2021). However, there are many gaps and deficiencies in the available data, and additional research is recommended. Conversely, some studies in mice found that inflammation resulted when the mice were exposed to MP subcutaneously (Rahman et al. 2021).
It has also been hypothesized that the microbes found on MP may cause adverse effects on the mammalian immune system. Communities of microbes can form biofilms on MP in the environment. For example, Escherichia coli has been found growing on plastic debris (Hirt and Body-Malapel 2020). These biofilms have been found to differ in their microbial composition relative to biofilms formed on natural substrates. In fact, human pathogens (Pseudomonas monteilii and Pseudomonas mendocina) have been detected in MP biofilms, which indicates that they could serve as vectors for pathogens (Hirt and Body-Malapel 2020). Reports directly related to humans, however, are scarce.
In a recent review of in vivo nonhuman mammalian studies with ingestion-based exposure to PS spheres less than 20 µm in size, Coffin (2022) provided a discussion of the potential for reproductive effects related to MP. Reproductive effects related to reduction in number and viability of sperm, markers of inflammation and oxidative stress in ovaries, and other reproductive responses were reported in several studies, but the effects could not be confirmed or quantified due to deficiencies and gaps in study design and reporting. Additional research was recommended
4.5.2.2 Health Effects Due to Inhalation
Bivalves (clams, mussels, or oysters) filter MP from the water and accumulate them easily, so they are commonly examined as quantitative aquatic exposure models in looking at human exposures to MP through inhalation, ingestion, and dermal absorption. Some of the data collected on these species can be related to calculate human exposure (Zarus et al. 2021).
For inhalation exposure, uptake of MP through the lung alveoli is likely to be highly dependent upon the physical/chemical characteristics of the MP particle. Lehner et al. (2019) suggested that particle sizes less than 1 µm can easily enter lung alveolar cells. Vethaak and Leslie (2016) reported that MP entering the respiratory system can be engulfed by macrophages via an immune response and are translocated to the lymphatic system.
Exposure to fine particles through inhalation has been demonstrated to trigger inflammatory and immunological responses in humans based on particle size (Chang 2010). For example, particles larger than 10 μm were found to be unlikely to penetrate and deposit into the lower respiratory tract and are cleared by nasal and airway ciliary mucosa, whereas smaller particles have been found to be more likely to penetrate the more distal parts of the respiratory tract (Chang 2010).
Inhalation exposure to airborne suspended MP and microrubbers is thought to result in inflammation of the respiratory system. Prata (2018) reported studies demonstrating that humans exposed to high concentrations of MP in certain industries (that is, the synthetic textile industry; the flock industry; and the vinyl chloride (VC) and PVC industries) have developed respiratory disease. A single histological study identified the presence of polymeric and cellulosic fibers of up to 135 µM in length in human lung tissue and may suggest these fibers are difficult to clear (Pauly et al. 1998). Interaction between chronic exposure to microfibers or microparticles and the lung may contribute to pathogenesis through chronic inflammation, irritation, and immune-related DNA damage (Greim et al. 2001, Prata 2018).
A review by Prata (2018) hypothesized that chronic inflammation and irritation due to MP inhalation exposure might promote cancer due to immune-related DNA damage. Similarly, (Chang 2010) reported that oxidative stress and chronic irritation due to NP may release pro-inflammatory mediators that induce angiogenesis (growth of new blood vessels from the existing vasculature), which, in turn, cause the formation and progression of malignancies. However, thus far, no studies are available to demonstrate whether chronic exposure to MP or NP (via inhalation or oral exposure) might increase cancer risk to humans
4.6 Ecological
Freshwater, marine, and terrestrial organisms can be exposed to MP through ingestion, dermal contact, and inhalation, with ingestion being the primary route of exposure. MP can be ingested at different trophic levels by both terrestrial and aquatic organisms and may accumulate through the food chain (Krause et al. 2021, Monikh et al. 2022). MP exposure can result in both physical and chemical impacts to organisms. Generally, smaller MP (<83 µm) have the ability to translocate across epithelial barriers while larger and smaller particles may impact nutritional endpoints through food dilution and other physical mechanisms (Figure 4-3). This section will cover the routes of exposure and general ecotoxicity of MP to aquatic organisms and ecological effects of MP to aquatic and terrestrial organisms.
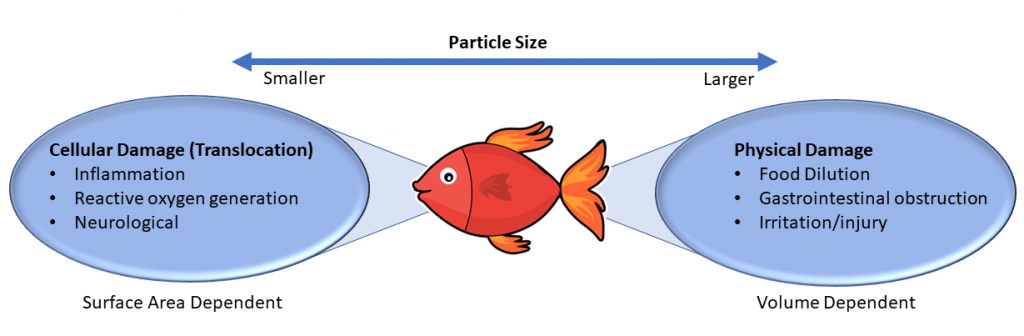
Source: Microplastics Team, created using concepts described in Mehinto et al. (2022)
4.6.1 Exposure and General Ecotoxicity
Exposures to MP present in the environment have different and varying ecotoxicological implications, including physical, chemical, and biological effects (Pereao, Opeolu, and Fatoki 2020). Documented effects are dependent on specific variables, including (1) the size, polymer types, shape, and concentrations of particles, and (2) the characteristics of the organism of interest, including its feeding strategy and other physiological characteristics, such as gill architecture in bivalves (Huang, Tao, et al. 2021, Ward et al. 2019). Research on MP assessment of risk can be a complex process, creating specific new challenges to environmental scientists, ecotoxicologists, and risk assessors (Catarino et al. 2021, Coffin and Weisberg 2022, Koelmans et al. 2022, Mehinto et al. 2022).
MP have been documented in nearly every environmental compartment (see Section 2). As a result, wildlife will encounter these particles through foraging, respiration, or other activities. Physical impact can occur through direct contact (that is, MP getting stuck in invertebrate appendages) or through ingestion, where MP can obstruct an organism’s digestive tract, which may lead to physical damage and prevent the uptake of nutrients (de Ruijter et al. 2020). Chemical composition of polymers, leaching of additives, or environmental chemicals adsorbed onto the MP can result in effects to wildlife. MP can adsorb heavy metals, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and various contaminants of emerging concern—for example, polybrominated diphenyl ethers and dichlorodiphenyltrichloroethane (DDT) (Antunes et al. 2013). It is not yet clear how significant these exposure scenarios are and how risk can be quantified (particularly for particles that may translocate), but they may have a minor contribution to effects (Koelmans et al. 2022).
Risk-based management tools include the Toxicity of MP Explorer (ToMEx), a living database that includes MP toxicity data to date (Thornton Hampton et al. 2022). ToMEx is discussed further in Section 4.7.3. Although the mechanisms of specific MP toxicity are not well understood, there is a strong weight of evidence for food dilution and moderate evidence for inflammation and oxidative stress based on translocation of smaller particles. MP introduce several unique exposure routes that likely have their own mechanisms related to their ability to impact wildlife, including particulate effects (that is, shape effects), the toxicity of monomers or additives as they are released, and the toxicity of adsorbed contaminants.
4.6.2 Ecological Effects
The primary ecological effects identified to date include molecular/cellular damage as a result of particles that are translocated into an organism, as well as food dilution as a result of MP ingestion. The size of particles and the physiology of the organism impact how and to what degree these endpoints are presented. This section includes a selection of the ecological effects of MP as they relate to the exposure of aquatic and terrestrial organisms to MP and NP.
4.6.2.1 Aquatic
Fish and other aquatic organisms can be exposed to MP incidentally through a contaminated food source or directly through respiration from the water column. Although fish and shellfish are known to ingest nondigestible material, including sediments of various sizes, shell fragments, fish scales, and bones (Hyslop 1980), MP differ from typical materials that are incidentally ingested due to their varied sizes, chemical composition, and ability to transport persistent organic pollutants. Generally, smaller MP tend to cause a greater number of documented impacts (Jacob et al. 2020), while fibers and fragments appear to have more harmful impacts than spheres (Gray and Weinstein 2017).
de Ruijter et al. (2020) conducted a metadata analysis of the different endpoints and exposure regimes for wildlife to MP. Their results were grouped based on the number of studies that examined specific endpoints. The most common suite of endpoints included effects in the alimentary tract (nutritional) and physical damage. Because MP are primarily ingested (and often egested), impacts to the gastrointestinal system are of particular concern. Significant changes in enzyme activity and intestinal permeability were observed in several of the studies. Other impacts included damage to the intestinal epithelium, erosion of the intestinal villi, and numerous liver pathologies (Jabeen et al. 2018).
Microplastics in the environment may also leach additives or otherwise contribute to chemical exposures to aquatic wildlife. Tire wear particles (TWP) have been recently targeted for their role in the release of 6ppd-quinone (see Appendix A.5), a chemical that has been found to be particularly toxic to coho salmon (Tian et al. 2021). Additional studies have investigated the acute toxicity of TWP and leachate to other species, including Pimephales promelas (fathead minnow)and Hyalella Azteca (an amphipod) (Chibwe et al. 2022, Halle et al. 2021); however, one study into benthic macroinvertebrates did not find significant impacts between exposures (Carrasco-Navarro et al. 2022). It is important that future studies involving TWP and other MP leachates characterize the contaminants associated with the exposure to improve comparability of results.
Another concern for MP contamination includes the transfer of adsorbed contaminants throughout the environment, as MP readily sorb chemicals in the environment and may transport these contaminants into biota if they are consumed. However, the concentration of sorbed chemicals may be a minor route of exposure when total environmental exposure is considered (Koelmans, Diepens, and Mohamed Nor 2022). Rochman et al. (2013) conducted a study comparing virgin MP to MP intentionally left in San Diego Bay for 3 months to accumulate POPs. Medaka (Oryzias latipes) were exposed to control and weathered MP. The cohort exposed to the weathered particles showed increased liver damage, including depleted glycogen, fatty vacuolization, and single-cell necrosis (Rochman et al. 2013). Bucci et al. (2022) exposed embryonic stage fathead minnows to “preconsumer” and environmentally collected MP. The results revealed significant differences in growth and deformity metrics. Field-collected particles were not chemically characterized, but they were analyzed as “environmental MP” for comparisons in this study.
Filter-feeding organisms, such as shellfish and daphnids, are known to ingest MP as part of their normal feeding strategy. Based on the available data, filter-feeding organisms, notably shellfish (such as oysters) and certain species of fish that target particles in the small micrometer size range, may be particularly susceptible (Huo et al. 2022, Jacques and Prosser 2021, Ward et al. 2019). Juvenile life stages of fish and smaller species also may ingest small particulates after confusing them with food items. There have been a few studies on freshwater filter feeders, such as daphnia, that have shown some responses to MP in freshwater invertebrates.
The mechanisms driving MP toxicity and the effects of MP are still not well defined and identified. Toxicological insight and awareness of MP will require filling knowledge gaps of combined toxicity (Du et al. 2021, Thompson et al. 2009). There is an increasing need to understand the environmentally applicable concentrations in which MP may affect or impact aquatic organisms, as well as studies on dose-response relationships. Recent advances in risk-based MP management provide a concise and particle-based approach to looking at MP toxicity based on the types of plastics and their resultant effects (Mehinto et al. 2022). For example, endpoints associated with food dilution have been attributed to the volume of MP consumed, whereas reactive oxygen and inflammation responses are best associated with surface area of particles, relating to their ability to cross epithelial barriers (Mehinto et al. 2022)
4.6.2.2 Terrestrial
Terrestrial toxicity testing has mainly been limited to soil invertebrates, plants, and microorganisms such as bacteria and fungi (Huo et al. 2022, Jacques and Prosser 2021). This section will focus on soil invertebrates.
The effects of MP on soil invertebrates have been studied using single-species tests that include representatives from different trophic levels and with different feeding strategies: earthworms (Eisenia fetida, E. andrei, Lumbricus terrestris), enchytraeids (Enchytraeus crypticus), springtails (Folsomia candida), oribatid mites (Oppia nitens), and woodlice (Porcellio scaber). The effects of MP were recorded at several organizational levels—that is, from cellular level, tissue level, whole-organism level, up to the level of population.
4.7 Risk Assessment Methodologies
Risk assessments depend on reliable exposure assessment and dose-response assessment for the receptor types and exposure settings being assessed. Hazard and dose-response assessment for MP to humans and the environment are challenging due to variability in the physical and chemical properties such as size, shape, polymer type, color, aging, hydrophobicity, density, surface area, and crystallinity (Chubarenko et al. 2016, Lambert, Scherer, and Wagner 2017, Rochman 2015, Rochman et al. 2019, Wang et al. 2018), and associated chemicals. Methodological and analytical variability and limitations have limited the development of consensus exposure data for most settings.
The Science Advice for Policy by European Academies (SAPEA) working group examined literature related to human health and ecological risks and concluded that “the risks of MP are still uncertain and the likeliness of the conclusions to be true is evaluated differently among experts” (SAPEA 2019). The absence of reliable analysis of concentration-dependence of risks was noted, as was the fact that provisional examples of risk characterization were available only for the aquatic compartment. Limited dose-effect data and inadequate exposure data were noted for both human health and ecological receptors. More recently, SCCWRP (2021) provided an updated assessment of potential risks for aquatic receptors and for human exposures via drinking water, noting that significant data gaps remain. Recent publications—Coffin, Weisberg, et al. (2022) and Mehinto et al. (2022)—are summary reviews of available studies that were conducted to determine the feasibility and confidence for deriving human health and aquatic ecosystem threshold values. The conclusions of the reviews are recommendations for research and evaluation to further the confidence in developing thresholds.
As noted above, MP literature continues to expand. The sections below focus on key data gaps in human health and ecological risk assessment to assist the reader in tracking the literature going forward
4.7.1 Human Health Risk Assessment
Due to the diversity of sources, a variety of human health risk assessments (HHRAs) are likely to be developed for settings in which MP are encountered by people. Product registration programs and federal and state drinking water regulations are likely to drive the development of HHRAs for personal care products and drinking water, respectively. Organizations that oversee food safety, such as FDA and the European Food Safety Authority (EFSA), are taking an interest in MP in foods, beverages, and food packaging. A regulatory driver for MP in indoor air is not apparent but is an area of research interest. Consequently, we are likely to see multiple HHRAs being developed by many organizations for these different sources.
Similarly, BfR (2020) undertook a data mining approach to synthesize knowledge on the occurrence and possible toxic effects associated with MP exposure via food products and beverages. The goal was to provide a basis for risk assessment and to identify important research gaps. The conclusion was that HHRA for MP is still not possible (Paul et al. 2020).
Dose-response assessment for MP HHRAs is in the very early stages of development, and exposure assessments are not yet considered to have produced consistent, reliable dose estimates (SAPEA 2019). One novel risk assessment framework assesses the different effects of the physical properties (for example, size, shape, etc.) and chemical properties (for example, polymer type, additive types, etc.) and develops dose curves for each unique mode of action. Through the use of particle density functions and statistical analysis, the potential risk of MP in the environment may be determined (Koelmans et al. 2022). Although this framework is equally applicable to human health and environmental risk assessment, it is still in the development stage and requires further refinement before being used in a regulatory context. Nevertheless, preliminary efforts to characterize MP health risks provide helpful insights.
The California State Water Resources Control Board has developed a framework for health-based guidance levels for MP in drinking water. Coffin, Weisberg, et al. (2022) reviewed 66 mammalian toxicity studies reporting adverse effects of MP and completed a screening to assess studies with data fit for use in deriving a drinking water effect threshold. It was observed that monodisperse particles with questionable relevance to most MP were used in studies; particularly, PS spheres between 5 nm and 20 µm dominated the prioritized studies. Although the available data were not judged adequate to develop health-based guidance levels, this effort resulted in recommendations of concentrations for toxicity studies and water volumes to support analytical goals for monitoring. The identified key data limitations included an inadequate effects database (too few doses, poor particle characterization, few endpoints tested, limited polymers, shapes, and sizes tested), lack of effects mechanism information to provide a basis for extrapolating to diverse particle types, and incomplete exposure data. Despite these limitations, a nonregulatory screening level was derived for water of 6.4 ng/L (applying a relative source contribution of 20% and a California-specific water intake of 0.053 L/kg-day). This would equate to 1.2 particles/L for this particle size (Coffin, Weisberg, et al. 2022). Note, these values may be updated in the future.
4.7.2 Ecological Risk Assessment (ERA)
To date, preliminary ERAs for MP have focused on exposures of aquatic organisms (Adam, von Wyl, and Nowack 2021, Brander et al. 2021, Everaert et al. 2018). As described above, initial exploration of potential impacts to soil organisms and other terrestrial receptors has begun; however, ERAs for terrestrial receptors have not been widely attempted, nor have they been determined to be needed (Jacques and Prosser 2021).
Research is ongoing and progressing to address data gaps and to inform ERA methodologies. This includes efforts such as those by the International Council of Chemical Associations (ICCA), in which scientists considered the use of a proposed conceptual ERA framework and considered data needs (Gouin et al. 2019). Recent publications have identified potential frameworks that could be implemented for ERAs of MP. Koelmans et al. (2022) published a generic framework that also combines the physical characteristics of particles to describe likely adverse effects. This framework incorporates the use of probability density functions to describe toxicologically relevant particle characteristics, use of quality assurance/quality control methods to assess if exposure and effects data are fit for use, and use of a calculation framework to assess exposure to plastic-associated chemicals through all relevant pathways. More recently, the SCCWRP engaged in the development of tools to derive species sensitivity distributions (SSDs) from a living database that includes MP toxicity data to date (SCCWRP 2022a). The Senate Bill SB 1422 supports the effort to develop a risk assessment framework by 2026, recently published in Mehinto et al. (2022). This framework identifies four critical management thresholds, ranging from low concern to high concern, where pollution control measures can be used to mitigate environmental emissions. These four threshold categories were derived using an SSD approach. Critical thresholds were developed for two effects mechanisms: food dilution and tissue translocation
4.7.3 Risk Assessment Tools
Despite the studies reporting adverse effects due to MP exposure, the current understanding of MP toxicity is limited. ToMEx is a tool that compiles and synthesizes data from different studies so that the data can be interpreted or analyzed to support the development of health-based thresholds for human and ecological health (Thornton Hampton et al. 2022). ToMEx enables researchers to quickly identify, extract, visualize, and analyze data that are most relevant to their research questions. The ToMEx database is divided into two distinct parts: aquatic organisms and human health. There are currently 162 publications pertaining to toxicity of MP to aquatic organisms and 55 publications on toxicity to human health.
Figure 4-4 from Thornton Hampton et al. (2022) illustrates how 70 unique variables were either extracted or derived from each study, including experimental design, test organisms, biological effects, and particle characteristics of MP used. The studies were then scored according to technical and risk assessment quality criteria developed by de Ruijter et al. (2020), but with modifications to address the goals of the California Microplastics Health Effects Workshop (SCCWRP 2021). The ToMEx application also has a tool for visualizing the data in the database.
The ToMEx database may also inform future experimental designs, not only by aiding in the identification of research gaps as described above, but also by facilitating the selection of experimental doses and relevant toxicity pathways (Figure 4-4). Sections 4.7.1 and 4.7.2 provided a brief discussion on how the ToMEx database could be useful in developing human health- and ecological-based threshold criteria.
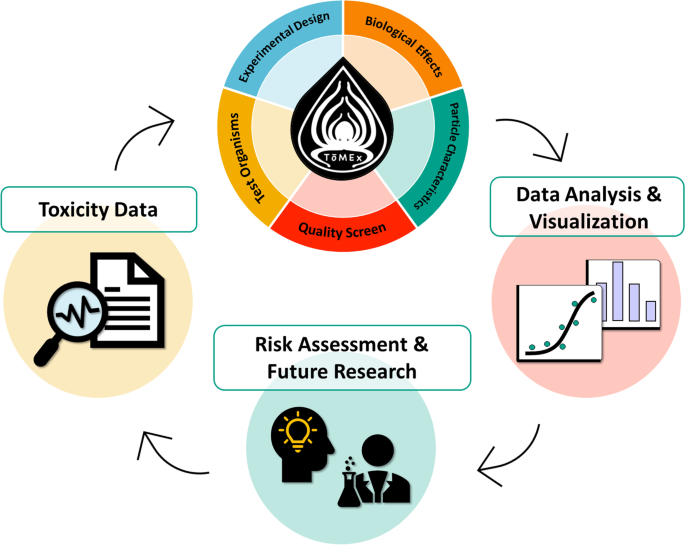
Figure 4‑4. Graphical abstract of ToMEx.
Source: (Thornton Hampton et al. 2022)
Similar tools have been developed for other environmental contaminants, such as the USEPA’s ECOTOX Knowledgebase (USEPA 2022d), an open access database that houses toxicity data pertaining to a wide array of contaminants and organisms.
These databases offer a significant advantage over traditional literature searches or mining data individually. The accessibility of ToMEx makes it a potentially valuable tool for collecting and analyzing data from hundreds of scientific studies and could advance scientific collaboration and policy decision-making.