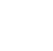1 Introduction
The overall goal of this section is to provide a primer on microplastics (MP) and introduce the various sections of the guidance. Click on any red dot in Figure 1-1 to read about MP in the environment.
Microplastics can be transported through atmospheric deposition
Discarded fishing line and nets and degradation of paint from vessels contribute to microplastic formation
Macroplastic trash may wash into the ocean, then break up into smaller and smaller pieces, which become microplastics
Industrial air emissions may contain microplastics
Manufacturing of primary microplastics such as nurdles to produce macroplastics can result in the release of microplastics to the environment
Microplastics can be released through wastewater outfalls and be transported by water
Microplastics can be released through wastewater outfalls and be transported by water
Aquatic organisms ingest microplastics, which can result in trophic transfer
Microplastics are generated through typical tire wear and breakdown of roadway materials
Microplastics may be present in agricultural lands due to direct application of fertilizer pellets, biosolids from wastewater treatment plants, or breakdown of plastic sheeting
Microplastics are generated by the breakdown of singe use plastics
Microplastics can be generated through the breakdown of rubberized play surfaces and plastic playground equipment or rubber mulch
Microplastics may be present in household products such as toothpaste or facial cleaners. Microplastics can be generated through household activities such as laundering of clothing
Microplastics have been found in snow on high mountain peaks
Microplastics are found in groundwater
Landfilling of macroplastics can lead to generation of microplastics
Microplastics are found in stormwater runoff
Figure 1-1. Microplastics in the environment.
Source: Jonathan McDonald
1.1 What Are Microplastics?
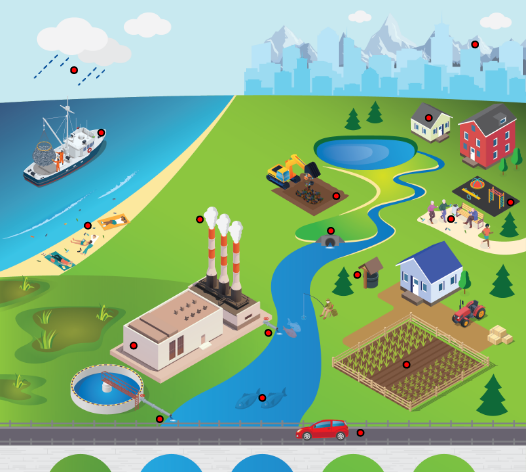
Various organizations, agencies, and researchers have defined MP in different ways. For the purposes of this document, MP are particles that are greater than 1 nanometer (nm) and less than 5,000,000 nm (or 5 millimeters [mm]) in their longest dimension and consist of solid polymeric materials to which chemical additives or other substances may have been added (CA SWRCB 2020). Polymers that are derived in nature (for example, cellulose, amber, proteins, wool, or silk) that have not been chemically modified (other than by hydrolysis) are excluded from the scope of this document. Plastic particles less than 1,000 nm in their longest dimension are also referred to as nanoplastics (NP). Although the definition of NP is still being debated, it is accepted in scientific literature that they are produced by the fragmentation of MP (or larger particles), measure between 1 nm and 1,000 nm in length, and demonstrate a colloidal behavior. Figure 1-2 shows the sizes of various items that fall within the MP size range, including a strand of DNA (approximately 2.5 nm), a red blood cell (7,500–10,000 nm), a fly ash particle (10,000–20,000 nm), the diameter of a human hair (60,000–120,000 nm), and a drinking straw (approximately 5,000,000 nm). This guidance document is not intended to specifically address NP that fall outside the range of MP but does provide limited further discussion of NP.
 Figure 1-2. Items comparable in size to microplastics.
Figure 1-2. Items comparable in size to microplastics.Source: Valerie Hanley
Although MP may be present in some consumer products, the bulk of MP in the environment likely result from the degradation of macroplastics. Primary sources of MP are materials that are manufactured using micro-sized plastic particles. Examples of primary sources or products containing MP include toothpaste or facial cleansers where microbeads are used as abrasives, makeup containing glitter, and as coatings on seeds, fertilizers, or pesticides. Secondary MP result from the breakdown of larger (macroplastic) particles or plastic products; their composition will be tied to the parent product or material.
Plastics are used in the manufacture of everyday products such as plastic bags, packaging, bottles, toys, food containers, cigarette filters, fishing line, rubber mulch, surface coatings, and other products. These materials when left outside or disposed in landfills may break down through chemical and physical processes, resulting in the formation of MP. As such, the visible plastic litter frequently observed along roadsides, in ditches, on beaches, or floating in water bodies, left untended, may eventually break down, resulting in the formation of MP, which are more readily transported through the environment than larger particles. Anthropogenic processes or actions may also contribute to the formation of MP in the environment. Two examples of such processes are the creation of MP from the wear of tires and the creation of MP during washing of clothing and textiles containing plastics.
1.2 History of Plastics
Human use of polymers dates to at least 1600 BC, but commercial production of plastics started in the 1920s and 1930s with widespread use outside of the military after World War II (Geyer, Jambeck, and Lavender Law 2017). Annual worldwide production of plastics increased exponentially from 1.5 million metric tons (MMT) in 1950 to 368 MMT in 2019 (NASEM 2022), with over 10,000 MMT of plastics produced worldwide since 1950. It is estimated that approximately 30 percent of all plastics ever produced are still in use today, while 60 percent have been disposed of in landfills or entered the environment, and the remaining 10 percent were incinerated or recycled (Geyer, Jambeck, and Lavender Law 2017).
The observation of small pieces of plastic debris in surface water, in the stomachs of birds, and in fish was first described in scientific papers published in the 1960s and 1970s (Carpenter et al. 1972, Colton, Burns, and Knapp 1974). However, the term “MP” was introduced only in 2004 (Thompson et al. 2004), and the issue of MP garnered widespread attention only in the 2010s as the result of ongoing scientific research and increasing attention to the impact of plastics on the environment. Monitoring of plastic debris in various parts of the world began in the 1990s; however, little long-term monitoring data exist specifically for MP to assist in assessing temporal changes. Okoffo et al. (2021) presented plastic prevalence data from archived biosolids collected from 1950 to 2016, providing one method that could be applied elsewhere to improve data on changes in plastic prevalence over time using archived samples. One potential problem with long-term comparisons across multiple data sets is the lack of standard methods for collecting and processing samples (see Section 3 for detailed information on sampling).
1.3 The Making of Plastics
1.3.1 Natural Plastics
Although many people associate the term “plastics” with synthetic plastics, plastics also occur naturally in the environment. “Plastic” is a broad term for materials that can be molded or shaped, and then hardened, maintaining their shape. Natural plastics, such as amber, animal horn, tar, latex, shellac, and cellulose, occur in a variety of different shapes, sizes, and colors (Science History Institute 2022). Cellulose, generally considered a natural plastic, can also be chemically modified to form semisynthetic materials such as rayon (Mendes, Prates, and Evtuguin 2021) or cellulose acetate.
1.3.2 Synthetic Plastics
Synthetic plastics are made from two main sources: fossil fuels and organic materials (Table 1-1). The majority of plastics are synthesized from petroleum products. Two common methods for synthesis of plastics from petroleum are addition polymerization and condensation polymerization. Addition polymerization consists of linking together monomers possessing double or triple bonds to form polymers. Condensation polymerization creates plastics via repeated condensation reactions between two different monomers (Science History Institute 2022).
Table 1-1. Common plastic polymers and their abbreviations
| Resin Code | Plastic Type Abbreviation | Plastic Type Name | Product Examples |
| PET | Polyethylene terephthalate | Water and soft drink bottles, salad dressing/peanut butter containers, rope, carpet, polyester fibers | |
| HDPE | High-density polyethylene | Milk jugs, juice bottles, freezer bags, trash bags, shampoo/detergent bottles | |
| PVC | Polyvinyl chloride | Plumbing and construction materials, pipes, liners, cosmetic containers, commercial cling wrap, siding | |
| LDPE | Low-density polyethylene | Squeeze bottles, regular cling wrap, trash bags, shopping bags, furniture | |
| PP | Polypropylene | Microwave dishes, medicine bottles, straws, ice cream tubs, yogurt containers, detergent bottle caps | |
| PS/EPS | Polystyrene/ Expanded polystyrene |
PS—CD cases, disposable cups, egg cartons, cutlery, video cases
EPS—Foam polystyrene, hot drink cups, |
|
| Other |
POM | Acetal (polyoxymethylene) | Fan wheels, gears, screws |
| PMMA | Acrylic (polymethyl methacrylate) | Aquariums, fiber optics, paint | |
| ABS | Acrylonitrile butadiene styrene | Car parts, Lego, wheel covers | |
| PA | Nylon (polyamide) | Air bags, clothing, thread | |
| P | Polyester | Fibers, rope | |
| PBT | Polybutylene terephthalate | Keyboards, relays, switches | |
| PC | Polycarbonate | Eyewear, safety helmets | |
| PEEK | Polyetheretherketone | Bearings, pump, pistons | |
| PE | Polyethylene | Mulch, housewares, toys | |
| PLA | Polylactic acid (bioplastic) | Packaging, syringes, textiles | |
| PSU | Polysulfone | Appliance parts, filters | |
| PTFE | Polytetrafluoroethylene | Teflon | |
| PUR, PU | Polyurethane | Adhesives, coatings, foams | |
| SAN | Styrene acrylonitrile | Brushes, hangers, printers |
When plastics are synthesized from organic materials, such as plants, they are called bioplastics (Science History Institute 2022). Bioplastics are currently less prevalent than traditional synthetic plastics. Bioplastics, by definition, are biobased, biodegradable, or both (Di Bartolo, Infurna, and Dintcheva 2021). Plastics that are biobased are derived in part or in whole from organic biological material (corn starch, cellulose, etc.). Some fibers, such as rayon, which has a cellulose base material that is dissolved from the original plant material and reprocessed into fibers, are considered semisynthetic plastics (Mendes, Prates, and Evtuguin 2021).
Many plastics start as a molecule of hydrocarbon and are then formed into chains using either addition or condensation polymerization (Science History Institute 2022). Plastics formed using addition polymerization typically can be softened by heating and hardened by cooling and are called thermoplastics. Plastics formed using condensation polymerization can be either a thermoplastic polymer or a thermoset polymer. Thermoset plastics retain their shape after forming, and after hardening, they cannot be remelted.
It is the linking of those molecules into polymers that gives each plastic type its unique properties. Polymers can form straight, branched, or crosslinked chains, depending on the type of chemical bonds that occur. Polymers can also have no defined molecular structure, called amorphous polymers, which are generally transparent and have a lower melting point. Polymers that are highly structured, called crystalline, are generally translucent and have a higher melting point (Science History Institute 2022).
Plastics that are biodegradable are capable of breaking down into natural materials (that is, carbon dioxide, water, organic matter), but are not necessarily biobased. Biodegradation of bioplastics requires that certain conditions be met (for example, temperature, humidity, presence of microorganisms), which is often only achieved by industrial composting plants. Access to composting facilities that are able to accept plastics is limited and varies by state (Figure 1-3). In the absence of those conditions, bioplastics can have the same environmental impacts as synthetic plastics, including the generation of MPs (Wang, Yu, et al. 2021).
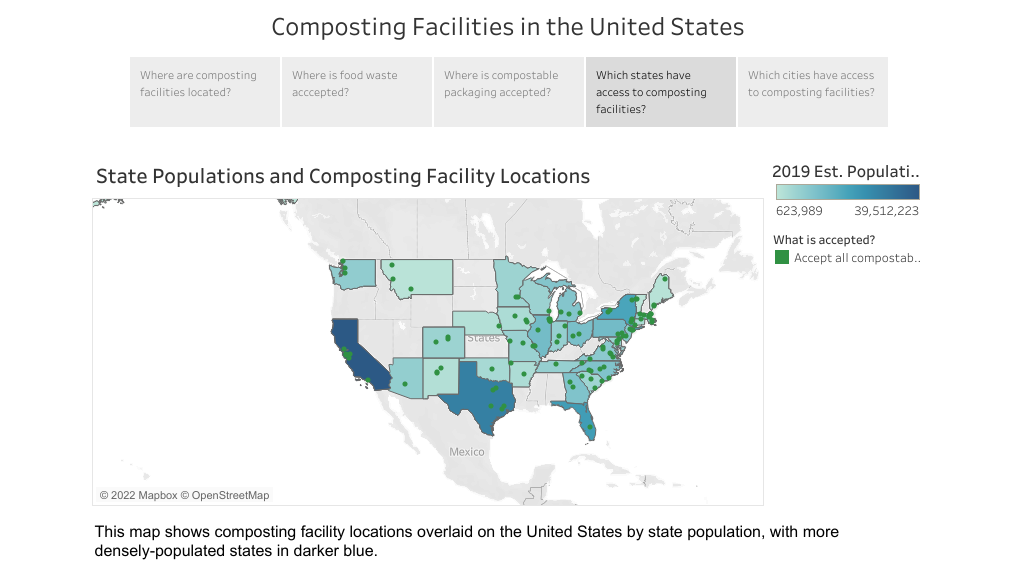 Figure 1-3. Map showing composting facility locations and state populations, with more densely populated states presented in darker blue.
Figure 1-3. Map showing composting facility locations and state populations, with more densely populated states presented in darker blue.Source: Greenblue.org
1.3.3 Physical and Chemical Properties of Microplastics
The diverse physical and chemical properties of MP impact their transportation, distribution, and degradation (Section 2), and their potential adverse health effects on human health and the environment (Section 4). Relevant physical properties include size and volume, shape, surface area, density, hydrophobicity, and crystallinity. Chemical properties include the polymer type, additives, surface charge, and sorbed substances.
Typical shape categories for MP include fibers (lines, filaments, and threads), films, foam, beads or spheres, pellets, and fragments (including powders and flakes) (Figure 1-4). The shape and structure of secondary MP are influenced by weathering and degradation—photo-oxidative, mechanical, and biological (Gray and Weinstein 2017)—and the amount of time the MP has been in the environment. The shape and structure of secondary MP may be indicative of the potential sources of the MP observed. For example, fibers tend to originate from clothing, textiles, or carpet; spheres may be microbeads from personal care products; pellets most commonly originate from raw materials used for plastic product manufacturing; and foam is often from expanded polystyrene (EPS) foam insulation or food packaging (Rochman et al. 2019). Plastic films are often reported on beaches and at the surface and bottom of water bodies; however, films are almost never reported among MP data, likely due to their breakup into threads and filaments (Chubarenko et al. 2016).

Figure 1-4. Various shapes, sizes, and colors of microplastic particles.
Source: Martindale, Weisberg, and Coffin (2020).
Particles with more irregular shapes may attach more readily to surfaces, including external and internal surfaces of living organisms (Lambert, Scherer, and Wagner 2017).
MP have a large surface area to volume ratio because of their small size. The proportional surface area increases as the MP particle size decreases. Typically, for a given mass of the same substance, spherical particles have the least surface area of all shapes, and fibers have the greatest surface area. As surface area increases, so does the adsorption of contaminants and fouling of the MP (Chubarenko et al. 2016). The adsorbed pollutants can subsequently leach back out into the environment or potentially cause health and ecological effects if ingested or inhaled. Additionally, biofilms, which may be toxic, may develop on the surfaces of MP and thus present additional exposure concerns.
The specific monomers, solvents, catalysts, and other additives used for the production of each type of plastic affect the physical and chemical properties of MP, as well as their reactivity and potential for sorption and leaching of chemicals. As a result, MP are increasingly being considered as a group of unique compounds, rather than a single compound.
1.4 Plastics Naming Conventions
Many types of plastics exist, each holding unique properties to fit a range of uses. A summary of common plastic polymers is provided in Table 1-1.
Synthetic fibers such as polyester, nylon, and acrylic are made from the same compounds used to produce plastics. Textiles made with these materials break down during production, care, and use, producing MP fibers. These fibers are included in the definition of MP.
1.5 Why Do We Care About Microplastics?
Microplastics are ubiquitous in the environment. They have been found in the air we breathe, the water we drink, and the food we consume. Microplastics pose a potential risk to humans and to wildlife through exposure to the chemicals in plastic and through physical impact (see Section 4 for detail). Humans may consume MP from many different sources—in products such as toothpaste that contain microbeads; in drinks stored in plastic bottles; in food that is packaged, stored, and microwaved in plastic; and in water from community water supplies or private wells, as well as other sources. MP are also consumed by animals and taken up in the roots and leaves of plants (Azeem et al. 2021), which, in turn, may be consumed by humans. MP may contain chemical contaminants or additives from the manufacturing process or adsorbed from the surrounding environment, as well as microbial pathogens from the environment (Cole et al. 2011, Yang et al. 2020). When MP are inhaled or ingested, any chemical additives or adsorbed contaminants are also inhaled or ingested. Plastics and MP can remain mixed with soil and sediments for years before their physical redistribution in stormwater runoff, agricultural integration/uptake, or human/animal consumption. Most plastics have the potential to degrade to MP; thus, today’s plastics could be tomorrow’s MP. The Pew Charitable Trusts estimated a 40% growth in plastics production over the next decade, thus concerns relating to MP are likely only to increase in the future (Lau 2021).
1.5.1 Additives
There are many different types of polymers used to create plastics (see Table 1-2) and each one has unique properties. Although the physical presence of MP presents environmental and health concerns, the substances and chemicals added to the plastics may be concerning as well. Some chemical additives used in plastics may cause physical effects or chemical effects. Additives, introduced during the plastic manufacturing process, can alter those properties to suit the requirements of a product. Common plastic additives can generally be grouped into four categories: colorants, fillers, reinforcements, and functional additives (Hahladakis et al. 2018). Detailed effects of MP (and their associated additives) on human health are discussed further in Section 4.
During the plastic manufacturing process, a small quantity of the additive(s) may be introduced. There are many different types of resins and polymers used to create plastics (see Table 1-2) and each one has unique properties. Common plastic additives can generally be grouped into four categories based on the effect to the resin: colorants, fillers, reinforcements, and functional additives (Hahladakis et al. 2018).
Table 1-2. Overview of common MP functional additives
Adapted from Bart (2005).
| Additive | Function | Example |
| Accelerators | Accelerate reactions in the manufacturing process to cross-link or break down polymers. | Also called activators, catalysts, compatibilizers, curing agents, initiators, promotors, scorch inhibitors or retardants, vulcanization accelerators, or vulcanization retardants |
| Acid scavengers | Neutralize acid residue from the manufacturing process. | Salts of weak acids |
| Adhesion promotors | Improve the adhesion of different materials (for example, polymers to inorganic substrates). | Also called coupling agents |
| Anti-blocking agents | Prevent or reduce plastic film from sticking together. | Lubricants, parting, or release agents |
| Anti-fogging agents | Prevent the formation of water droplets and increase the transparency and the polymer surface critical wetting tension. | |
| Antimicrobials | Protect plastic material against the growth of algae, bacteria, fungi, mold, etc. May include biocides and preservatives. | Used in PVC and polyurethane foams |
| Antioxidants | Inhibit the oxidation of organic materials in the manufacturing process; protect polymers, elastomers, foods, fuels, and lubricants. | Also called free radical scavengers, light stabilizers, peroxide decomposers, tackifiers, ultraviolet (UV) absorbers, or screening agents |
| Anti-radiation agents | Slow the degradation process resulting from gamma radiation. | Used in rubber industry |
| Anti-skinning agents | Control skin formation resulting from exposure to air. | Used in alkyd or oil paint |
| Anti-slip agents | Reduce surface friction, which allows one layer of film to slide easily over another. | Used in lubricants, slip agents, and surfactants |
| Anti-static agents | Reduce or eliminate electrostatic charge. | Used in static-free supermarket bags |
| Blowing agents | Generate or release gases into selected areas to create cellular structures during the manufacturing process. | |
| Bonding agents | Improve cohesion of two plastics. | Added to plasticized PVC so it can adhere to human-made fibers of polyester or polyamide to make waterproof fabric or tarps |
| Clarifying agents | Enhance the clarity, surface gloss or similar physical properties; may also be referred to as nucleating agents. | |
| Corrosion inhibitors | Remove active catalyst residues. | |
| Defoaming agents | Remove trapped gases from liquid mixtures during the manufacturing process. | |
| Degradation additives | Degrade plastic waste with biodegradation, chemical degradation, hydrodegradation, or photodegradation processes; includes photosensitizers and pro-oxidants. | |
| Diluents | Reduce viscosity while facilitating processing. | Used in epoxy resins |
| Driers | Accelerate autooxidation and hardening of coatings. | |
| Elastifying agents | Improve elasticity and cold temperature flexibility. | Used in cables, self-adhesive products |
| Exotherm modifiers | Reduce maximum temperatures reached during exothermic reactions. | |
| Flame retardants | Reduce flammability, slow down or disrupt ignition or smoke evolution, or alter the combustion process. Includes halogenated additives, intumescent additives, smoke suppressants, and spumific additives. | |
| Heat stabilizers | Prevent polymers from oxidizing and/or decompose oxidized products in the manufacturing process. | Also called process stabilizers |
| Impact modifiers | Improve impact strength and rigidness of the polymers. | |
| Initiators | Promote radical polymerization of polymers. | |
| Mastication agents | Lower viscosity by assisting in breaking molecular chains; includes peptizers and processing aids. | |
| Metal deactivators | Prevent metal-catalyzed oxidation during the manufacturing process. | Used in wire and cable applications |
| Odor modifiers | Mask undesirable odors or add scent. | |
| Photosensitizers | Increase the photodegradation rate by absorbing light. | |
| Plasticizers | Improve the flexibility of product. | |
| Processing aids | Coat the metal surface of a die, changing the interfacial properties between the metal and a polymer melt. | |
| Quenchers | Prevent polymers from degrading through photolysis. | |
| Shrinkage modifiers | Control shrinkage after molding. | |
| Thickening agents | Increase viscosity so that a liquid resin becomes a tack-free solid. | |
| Thixotropic agents | Modify viscosity or shear stress by producing low viscosity at high shear rates (for example, under kinetic conditions) or high viscosity at low shear rates (for example, under static conditions). | |
| Wear additives | Improve the ability of a base polymer to resist wear. |
Colorants, such as dyes or pigments, are chemical compounds that are used to alter the color of a polymer. The type of colorant that is used is based, in part, upon the type of polymer. For example, dyes, which are soluble colorants, are used to add color to polycarbonates (PC) and polystyrenes (PS), whereas pigments, which are insoluble, are used to add color to polyolefins. Use of specific colorants can also be based on the desired effect on the final product, such as fluorescent, metallic, pearlescent, phosphorescent, photochromic, or thermochromic, or the ultimate application or use of the end plastic, such as food, packaging, or toys (Hahladakis et al. 2018).
Fillers (sometimes called extenders, dispersing agents, homogenizing agents, or reinforcements) are inert material that add bulk to plastics, coatings, adhesives, and sealants (CROW 2022). In addition to providing a cost benefit by lowering manufacturing cost, fillers can also improve moldability and stability of the polymer, reduce thermal expansion, and increase the heat-deflection temperature (CROW 2022). Alumina trihydrate, barium sulfate, carbon black, calcium carbonate, calcium sulfate, clay, glass beads and fibers, kaolin, mica, and wollastonite are the most common additives used as fillers (CROW 2022).
Reinforcements used in plastics include carbon, glass, mica, aramids, and other materials in the form of particulates, fibers, mats, or fabrics that are added to plastics to increase strength or provide other beneficial physical traits (Rosato, Rosato, and Rosato 2004).
Functional additives are compounds that are added to plastics to enhance or alter existing properties of plastics or add new properties. These compounds are classified by the desired effect that the compound will have on the polymer. An overview of common functional additives is presented in Table 1-2.
Although the uses of plastic additives are regulated via many avenues, including under the U.S. Department of Agriculture for additives that may come into contact with food or beverage products (for example, 21 CFR Section 177), by the U.S. Environmental Protection Agency (USEPA) for additives considered toxic, and by Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) in Europe, such regulations are not focused on preventing adverse effects to the environment when plastics degrade or when additives leach from the plastics and enter the environment. Although the additives used in plastics manufacturing provide many benefits, these additives may also present potential toxic dangers and may contain regulated substances. As plastics slowly break down into the environment, the additives can leach out from the polymer/resin and mobilize into and migrate through the air, water, and soil. Leachates containing chemicals from plastics can accumulate or migrate in the environment; thereby magnifying the harmful effects of nano-sized plastic pieces by adding a toxic component (Tian et al. 2021). However, some studies have conflated the potential human health risk associated with consumption of species in which MP have accumulated as the chemical exposure risk is more so related to the chemicals that leach out of the MP rather than the MP themselves.
Because of the potential for human exposure, environmental persistence, and presence of chemical additives in MP, federal, state, and local authorities are looking to regulate MP (Sections 5.2.6 and 5.2.7).
1.5.2 Human Health and Ecological Effects
Exposure to MP may occur via ingestion or inhalation. The presence of MP in foods and beverages (including potable water), personal care products, and indoor and outdoor air has been well documented (Danopoulos et al. 2020a, Nicole 2021, Prata 2018, Revel, Châtel, and Mouneyrac 2018). Once taken into the body, adverse health effects may result due to the physical presence of MP, toxicity resulting from the presence of microorganisms concentrated on a biofilm surrounding the MP (Yang et al. 2020), chemicals adsorbed onto the MP, and/or direct toxic effects resulting from the chemical composition of the MP. The potential to cause human health or ecological impacts is influenced in part by the chemical and physical properties of the MP, including polymer type, particle size and shape, and the potential sorption of other chemical substances onto MP. The number of published studies showing adverse effects from MP exposure is increasing (Figure 1-5). Human health effects and ecological effects are discussed in greater detail in Section 4. Risk assessment associated with MP is challenging and is continuing to evolve. Conclusions derived from risk assessments of MP frequently lack consensus from the scientific community due to inadequate dose-effect and exposure data and associated data gaps.
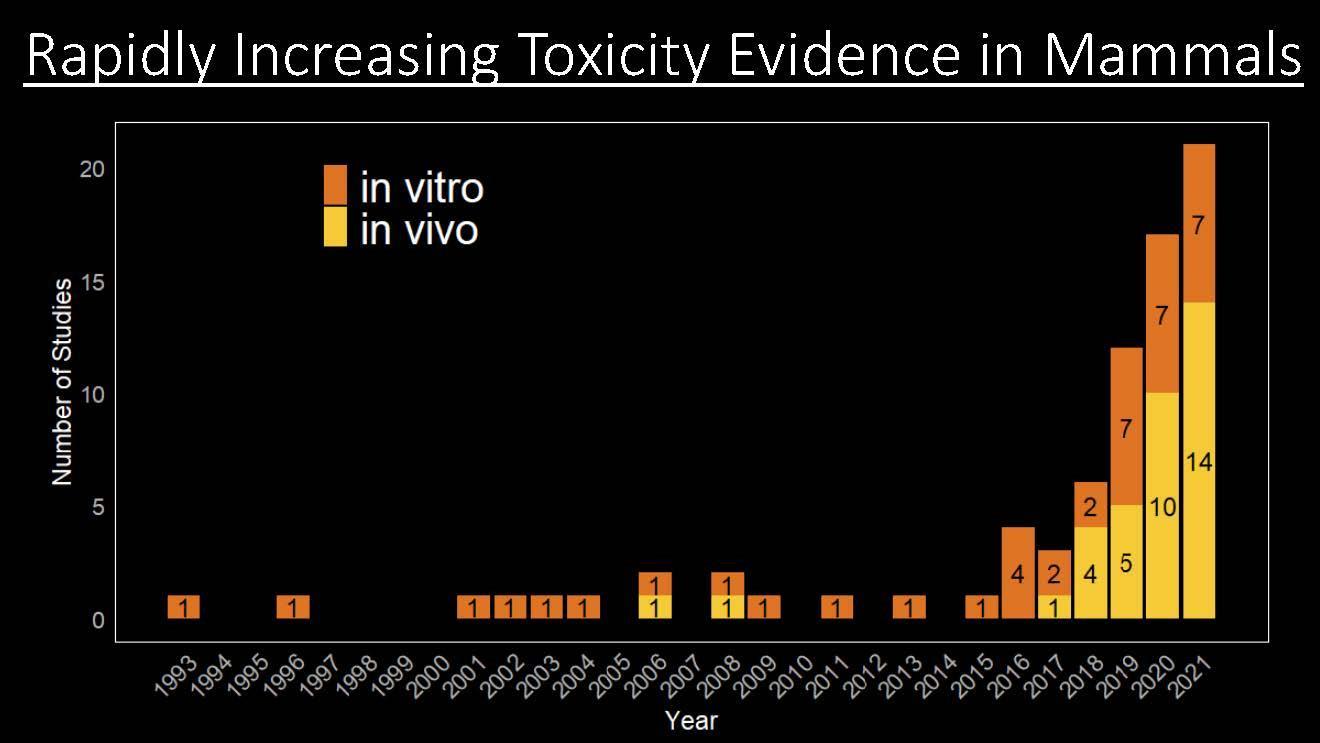
Figure 1-5. The number of studies showing evidence of toxicity in mammals is rapidly increasing each year.
Source: Coffin (2022).
1.6 Distribution of Microplastics in the Environment
1.6.1 Worldwide Distribution
MP appear to be ubiquitous in the environment and can be found around the world, including in ice from Antarctica and in sea salt (Auta, Emenike, and Fauziah 2017). MP have been identified in multiple media, including ambient air, surface water, groundwater, wastewater, wastewater biosolids, soil, sediment, and biota. The accumulation of MP varies geographically with many factors, including location, hydrodynamic conditions, environmental pressure, and time (Shahul Hamid et al. 2018).
Within the marine environment, MP appear to be generally present in greater abundance near the shore and close to urban areas than in waters located farther from developed areas (Bošković et al. 2022, Desforges et al. 2014), thus indicating a strong anthropogenic influence. Distribution of MP in nearshore areas is also affected by tides, currents, and rivers. The abundance of MP in the marine environment is particularly high in semi-enclosed, shallow marine waters such as the coasts of Louisiana, the northern Gulf of Mexico, and the Mediterranean Sea (Cózar et al. 2014). In more remote areas of the oceans, the distribution of MP is strongly influenced by currents, cyclones, and wind directions.
The presence of MP in freshwater bodies is largely affected by anthropogenic activity, with greater abundance of MP noted close to urban areas, wastewater treatment plants, or other sources. For example, a study performed by Estahbanati and Fahrenfeld (2016) documented significantly increased concentrations of MP downstream of wastewater treatment plants compared to upstream waters. Temporal variations in the amount of MP in fresh water and marine water have also been observed by many researchers with generally greater concentrations of MP observed during rainy seasons (Harris et al. 2022, Li, Wang, et al. 2021).
Studies also indicate rapidly increasing concentrations or abundance of MP in the environment. A study performed by Amélineau et al. (2016) measured the mean density of MP in the water column of Greenland and noted an increase from 0.99 items/m3 to 2.38 items/m3 from 2005 to 2014. Most MP research has been focused on the marine environment, but some studies suggest that the amount of MP released on land is up to 4–23 times the amount of MP released directly to oceans. MP accumulation on land is most significant in areas of strong anthropogenic influence, such as in urban areas (Horton et al. 2017).
1.7 Fate and Transport of Microplastics
MP may enter the environment via permitted or unpermitted point source releases, nonpoint source releases, or degradation of macroplastics. Point sources may include, but are not necessarily limited to, discharge of MP to surface water via outfalls, land application of biosolids or treated water containing MP, the use of fertilizers or pesticides containing micro-sized plastic particles, or discharge of air emissions containing MP. Nonpoint discharges include stormwater runoff, release of MP due to tire wear, atmospheric transport and deposition from nonspecific sources, degradation of macroplastics, or other mechanisms that are not tied to a specific source (See Appendix A.3). Although releases of primary MP do occur (see Appendix A.4), the degradation of plastic products and plastic wastes in the environment is considered a significant contributor to the volume of MP in the environment (Ivleva, Wiesheu, and Niessner 2017, Zhang, Hamidian, et al. 2021). The formation of MP from macroplastics may occur via physical and chemical weathering, but anthropogenic activities, such as laundering of textiles containing synthetic fibers, may also contribute MP to the environment.
Weathering processes may affect the plastic properties, making it difficult to trace MP to original sources and definitively determine their mechanism of release or pathway to the environment. Nevertheless, it is important to understand the original products and sources of MP to identify ways to reduce or eliminate the introduction of MP into the environment. The environmental fate and transport of MP are discussed further in Section 2 of this document.
As discussed in Section 1.3, the characteristics of MP particles may affect their transport in the environment. For example, the density of MP particles ranges from <0.05 g/cm3 for PS foam to 2.3 g/cm3 for PTFE and affects their transport in the environment. Low-density MP (for example, foamed PS, PS) float on water surfaces and are exposed to winds, waves, and currents, with wind being the most important factor for transport. As such, low-density MP may travel rapidly in the environment and may be transported long distances. By contrast, high-density MP tend to be less mobile in the environment. Low-density MP are also found in sediments, suggesting that biofouling causes an increase in density and contributes to a loss of buoyancy and sinking (Chubarenko et al. 2016). Recent studies have shown higher proportions of high-density polymers in offshore waters, suggesting that high-density MP can travel greater distances in surface waters than previously thought (Eo et al. 2021).
1.8 Environmental Justice and Microplastics
Environmental justice (EJ) is the “fair treatment and meaningful involvement of all people regardless of race, color, national origin, or income with respect to the development, implementation and enforcement of environmental laws, regulations, and policies” (USEPA 2021b). Although further research is needed to fully understand the health impacts of MP in general, and on specific subpopulations, the topic of EJ as it relates to marine litter and MP is of growing concern (UNEP 2019). On local to global scales, the current efforts to adequately manage and decrease plastic pollution are insufficient to address the specific needs of various groups who are already in vulnerable situations, and are socially, economically, politically, institutionally, or otherwise marginalized (UNEP 2021a).
The United Nations (UN) reports marine litter, including MP, as a major EJ concern. “Vulnerable communities, including those who depend on wild seafood for their diet, face greater risks to their health and livelihoods from marine litter and microplastics.”(UNEP 2019) Each year, around 8 million metric tons of plastic are dumped into the ocean; MP account for approximately 3% of this pollution but have the potential to have significant impacts on human health (PEPH 2020). In some countries, such as Fiji, up to 80 percent of land-based litter ends up in natural waters. Other developing countries, like Haiti, have no wastewater treatment and limited waste management options
1.8.1 Impacts over the Plastics Life Cycle
When considering the impacts of MP on various populations, it is critical to consider the full life cycle of plastics from production to consumption to disposal, as well as the fact that the responsibility of contamination spans a complicated range of stakeholders that includes plastic producers, manufacturers, consumers, waste handlers, and governments (Eriksen et al. 2018). Therefore, the full cost of plastics must consider the range of social and EJ concerns as inequitable burdens of environmental and health impacts imposed on communities across the life cycle of plastics (see also Section 6.1 for additional details). Further, waste reduction and materials management strategies should be inclusive of local communities with EJ concerns (USEPA 2021c). Illustrative examples of EJ considerations across the plastics life cycle and population groups at increased risk are provided in the following sections.
1.8.1.1 Production
The vast majority of plastics are produced from petrochemicals, which are sourced from fossil fuel extraction through mining, fracking, or drilling of coal, oil, and gas; further, due to the localization of fossil fuel production and the logistic complexities of transport, plastic production is often concentrated in regions where fossil fuel development is present (CIEL 2017, UNEP 2021a). Extraction and refining of fossil fuels, such as hydraulic fracturing for natural gas, may release toxic substances into the air, water, or soil and impact nearby communities (CIEL 2019). Fossil fuel exploration and development have impacted indigenous peoples around the world related to land tenure (UNEP 2021a). Potential occupational exposure across the plastics supply chain, particularly among industrial workers, should also be considered, as studies have indicated occupational exposure to airborne MP in workers of synthetic textiles and the vinyl chloride or PVC industries (Prata, da Costa, et al. 2020). Most communities next to industrial facilities in the United States are disproportionately occupied by low-income individuals and communities of color who have faced a legacy of systemic disadvantage and inequitable burden of health impacts resulting from environmental contamination (CATF 2017).
1.8.1.2 Consumption
The societal demand (by some) for single-use plastics and corporate decision-making relating to, for example, excessive packaging, further increases the plastic burden, resulting in greater volumes of plastic requiring disposal and increased plastic production (NASEM 2022). Regulations limiting MP have focused primarily on single-use plastic bans (Karasik et al. 2020) and primary MP legislation (Congress.gov 2015). Specific regulations are included in the regulatory context section (Section 5).
There have been efforts in some communities to ban or disincentivize single-use plastics, and there is a recent push to more circular economy efforts, which could include consideration of the use of sustainable alternatives and improved collection and upcycling of plastics, going beyond recycling efforts to maximize the value of products and materials for as long as possible and avoid potential contamination, toxicity, and pollution (Bucknall 2020). However, sustainable or green alternatives are not always equally accessible to all populations, including low-income populations.
1.8.1.3 Disposal
Given existing solid waste management infrastructure and practices, plastic waste is typically managed by being disposed of to a landfill, incinerator, or recycling facility. Most often, such facilities are sited in economically disadvantaged communities (The New School 2019). The current suite of plastics waste management technologies, including incineration, co-incineration, gasification, and pyrolysis, may result in the release of toxic metals, gases, and substances into the air, water, or soil, and thereby may lead to either direct or indirect exposure to frontline or fence line communities (CIEL 2019).
Poor solid waste management practices can contribute to the release of plastics and MP to the environment. In developing and low-income countries, waste management challenges or gaps (for example, the lack of adequate solid waste collection and management) can result in greater mobilization of plastics to the environment, and thus, greater MP exposure for these populations.
1.8.2 Effects on Sensitive Populations
Certain portions of the population may be more susceptible to potential health effects resulting from exposure to MP. These include women, communities that rely on subsistence fishing/aquaculture and farming/agriculture, children, those relying on aquatic ecotourism, residents of countries with less developed waste management programs, individuals living closer to sources of MP, and individuals who rely more heavily on plastic products due to lack of choice or due to convenience.
- The nexus between the plastics life cycle and socioeconomic factors has myriad direct and indirect effects, including ecosystem devaluation and loss of tourism (NASEM 2022), particularly for island and coastal communities. Communities, including indigenous groups or those relying on wild seafood or marine mammals for their diet, may face greater risks to their health and livelihoods from marine litter and MP, and the chemicals that may leach out of plastics, which can be more concentrated (UNEP 2021a).
- With regards to exposure from household sources, women face a higher burden of exposure to plastics and MP due to differences in biology, social roles, and political power in regulating plastic use and health standards. Plastics have become more prevalent in our daily lives and are encountered more frequently through items such as cosmetics, personal care, and feminine hygiene products (UNEP 2021a).
- Physiological and behavioral differences in children may also result in greater exposure in some instances. Recent studies discovered that infants have 10–20 times higher concentrations of MP in their feces than adults (Zhang, Wang, et al. 2021), and including meconium and placenta (Braun et al. 2021, Ragusa et al. 2021). Recent studies have also demonstrated the presence of MP at playgrounds, which can be attributed to the deterioration of plastic play equipment (Koutnik et al. 2023) and rubberized play surfaces (Olshammar et al. 2021) The potential impacts of MP on children and the uncertainty regarding lifetime accumulation of MP are unresolved and require further research.
1.8.3 Environmental Justice, Taking Action
EJ issues with complex environmental challenges such as plastic pollution are difficult to address because they often transcend environmental, public health, human rights, and other boundaries (Osofsky 2004). Nevertheless, EJ considerations must be considered when assessing the health and socioeconomic impacts of MP. The UN recommends that countries develop and implement programs focused on environmental conservation and creation of awareness of the negative effects of MP. The UN also recommends that governments seek the opinions of indigenous peoples when developing environmental protection policies and provide transparency to all stakeholders, but especially to affected communities (UNEP 2022a). The USEPA established America’s first national recycling strategy for plastics in 2021, noting the production of about 300 million tons of plastic waste annually around the world. The draft USEPA plan acknowledges EJ concerns and places priority on addressing the impacts of recycling on poor and minority communities (USEPA 2022e). More specifically, the plan calls for the transformation of waste and materials management in the United States, including actions to address challenges facing the recycling system, both domestic and international, and engagement of community and tribal stakeholders in affected communities.
“We recognize the burden that living near waste and waste-related facilities can have on communities when waste is not properly managed, which can lead to higher levels of chronic health values.”
1.9 Acknowledgment of the Evolving State of the Science
MP are considered an emerging contaminant of concern and as such, the state of the science is evolving rapidly. The USEPA acknowledges that MP are an emerging contaminant that may pose human health and environmental risks (Potter 2021). Challenges relating to MP include the following:
- Difficulty in comparison of MP studies. Focus on MP has increased significantly since 2004, with the number of publications relating to MP increasing from no publications in 2004 to over 1,700 publications in 2020 (Qin et al. 2020). Researchers have found that, as with many developing disciplines, the reliability of study findings in publications relating to MP studies is significantly variable. Comparison of data between studies has been complicated by variability in how MP are defined, a lack of standardized test methods, and even differences in the units used to report the presence of MP in various media. As such, researchers, government officials, media, and others are challenged with verifying the reliability of publications and data relating to MP.
- MP fate and transport. Additional study is required to better understand the environmental fate and transport of MP (see Section 2 for more information).
- Representative sampling and analysis methods. MP sampling and analytical methodologies are still being developed and evaluated. Standardized sampling and analytical methods have not been adopted by many regulatory agencies. Also, synthesis of MP reference standards is necessary to support exposure studies. Sampling and analytical methods are discussed further in Section 3 of this document.
- MP toxicity and health effects. The toxicity of MP is not well understood, and limited information has been collected relating to long-term biological effects. Challenges relating to the current state of understanding of toxicity and human health effects are discussed further in Section 4 of this document.
- Determination of an appropriate framework for risk assessment. Further consideration of an appropriate framework for risk assessment associated with polymeric materials is needed to determine whether the risk associated with MP should be evaluated based on the risk associated with individual chemicals or whether a different approach would be more appropriate. Risk assessment approaches are discussed further in Section 4.7 of this document.
MP can result in complex and wide-ranging physical and chemical effects but understanding of long-term biological effects is very limited. Further, in combination with climate stress, MP may result in multi-stressor impacts, particularly in densely populated urban areas; these stresses may affect the long-term health and resilience of ecosystems.