2 Environmental distribution, fate, and transport
To better understand the complexities of MP pollution, we need to understand its distribution, as well as its transport pathways and fate in the environment. Understanding these concepts is crucial in developing management strategies and policies to remediate and reduce MP pollution. Select a red dot on Figure 2-1 to learn more about the distribution, fate, and transport in various environmental media.
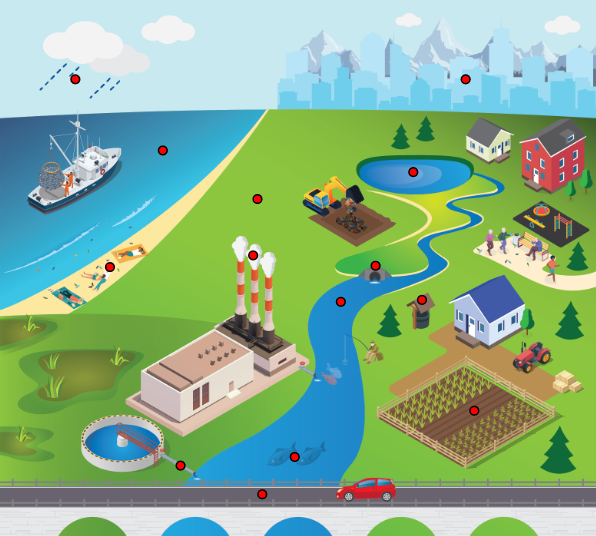
Figure 2-1. Site conceptual model for the environmental distribution of MP.
Source: Jonathan McDonald and the ITRC MP Team
Plastic waste and MP are found ubiquitously throughout the world, in soils, sediments, waters (oceans, lakes, rivers, groundwater), the atmosphere, and at the northern and southern extents of the planet in the polar ice caps. Plastic wastes enter the environment through various forms of disposal and incidental (both accidental and non-accidental) releases. Physical characteristics (for example, size, shape, density) and chemical components (for example, polymer type, additives) are critical in further understanding the distribution, transportation, and fate of MP in the environment.
Sources of MP can be broken into “primary” and “secondary.” Similar to other pollutants, MP can enter the environment through point or nonpoint sources.
2.1 Primary vs. Secondary MP
MP found in the environment can be classified as primary or secondary (Figure 2-2). Sources of primary MP include MP production facilities, as well as product manufacturing facilities where MP are used as components of industrial or commercial products. Secondary MP are generated through the physical, chemical, and biological alteration/degradation of larger pieces of plastic. Degradation of plastic wastes in the environment is considered to be one of the major processes contributing to the accumulation of MP in the environment (Ivleva, Wiesheu, and Niessner 2017, Zhang, Hamidian, et al. 2021). Other processes include the use and processing of plastic-containing materials, such as washing of synthetic fabrics (see Appendix A.6) and abrasion of tires on the road surface.
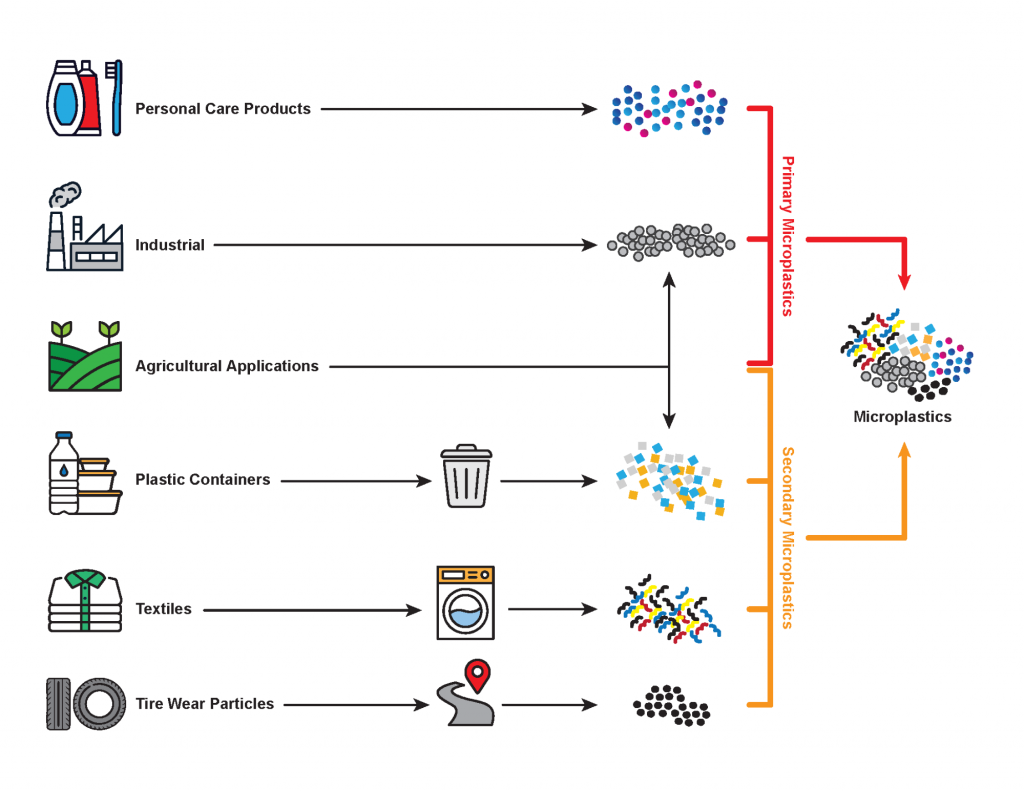
Figure 2-2. Primary and secondary microplastics and examples of associated sources.
Source: Johnathan MacDonald
2.1.1 Primary Microplastics
Primary MP are tiny plastic particles that were designed specifically for their use in industrial and commercial products. Examples include nurdles and fertilizer pellets. Primary MP can enter the environment through spills or other incidental releases during manufacturing or shipping, or in the waste streams associated with the manufacturing process. For example, MP can be released directly to the atmosphere through process air discharges or to water bodies as a component of industrial wastewater. Sources of primary MP are also found in personal care products and cleansers that contain MP beads as a component of the product (facial cleanser, toothpaste, and makeup), and can be released during or as a result of product use. Primary MP are applied to agricultural fields in the form of polymer-coated nutrient prills.
2.1.2 Secondary Microplastics
Secondary MP are generated through the chemical and physical degradation of larger plastic products already in the environment, such as through the breakdown of synthetic fibers and consumer products (for example, water bottles, plastic bags, cigarette butts), industrial/ construction products (for example, paints, plastic sheeting), washing and drying of synthetic textiles, tire wear, and recreational wastes (for example, fishing line). Synthetic fibers that have been found to be most common sources of MP include P, PE, PP, and PVC.
The plastic degradation process is important in determining the fate and effects of MP on the environment. The size distribution of plastic particles in the aquatic environment is impacted by a variety of factors, including biofouling (growth of a biofilm over the plastic, increasing its buoyancy or density, depending on its size and shape) and aggregation, flocculation, fragmentation, the time of residence, and transportation routes (Darabi et al. 2021).
Photodegradation is recognized as the most important process that initiates plastic degradation on the surface of land or water, whereas biodegradation by microorganisms is the main cause of plastic degradation in seawater in the aphotic (no light) zone (Khoironi et al. 2020, Zhang, Hamidian, et al. 2021). Although not all (Henry et al. 2018), most plastics are subjected to abiotic and biotic degradation processes involving chemical, physical, and biological reactions in the environment. Additionally, photodegradation has the ability to cause oxidation and chain scission (degradation of the polymer chain), forming low molecular weight degradation products and causing changes in physicochemical and mechanical properties (Zhang, Hamidian, et al. 2021). Other abiotic degradation processes (for example, heat, mechanical, chemical), along with biological degradation and disintegration, also convert plastic wastes into MP that are then distributed throughout the environment through various transportation vectors
2.2 Point and Nonpoint Sources
Although the discussion of sources of primary and secondary MP addresses the types of MP that are being released, point vs. nonpoint sources show us specifically where these releases are coming from. Point sources of MP enter the environment from a single place, while nonpoint sources of MP enter the environment from many different places at once. These MP are then transported throughout the environment through atmospheric and fluvial pathways. Wind and stormwater runoff are believed to be the main mechanisms that transfer plastics from land to water. Additionally, overland flow following precipitation events can transport MP from land application areas to nearby water bodies (Zhang, Ma, et al. 2021). Some MP can even migrate vertically along with the percolating stormwater and eventually reach groundwater, where they can be transported downgradient, away from the source area. This means that they can travel significant distances from their depositional origins and impact communities and ecosystems that are geographically removed from where they were originally released.
2.2.1 Point Sources
Point sources of MP include industrial and municipal wastewater outfalls, stormwater outfalls, and industrial smokestacks. Untreated outfalls can discharge MP directly to local surface water bodies where they can be transported downstream to lakes, estuaries, and eventually bays and oceans. In countries where wastewater is treated prior to discharge, 72% of MP on average is removed during primary treatment and 94% on average in wastewater treatment plants (WWTPs) where tertiary treatment is used (Iyare, Ouki, and Bond 2020). Mak et al. (2020) identified local MP in the sewage treatment and stormwater outfalls into Victoria Harbor, Hong Kong, at concentrations up to 10,816 pieces/m³. Plastics consisted mainly of PE and PP and were being discharged at an average rate of 3.5 mg per capita per day. Treilles et al. (2019) investigated MP in stormwater at the outlet of an outfall located in Sucy-en-Brie (Paris suburb, France). Preliminary results showed MP concentrations were in the range of 4,600–93,000 fragments/L, in which PA and PE predominated.
The MP removed through treatment end up in the biosolids, which are typically incinerated, disposed in landfills, or spread out to dry and mixed in with soils as amendments (for example, to agricultural fields). During the drying process, MP can be dispersed throughout the environment by the wind.
Within the marine environment, point sources of plastics include material lost or discarded from commercial and passenger ships, as well as aquaculture facilities (Karbalaei et al. 2018). Additionally, some marine paints contain synthetic polymers that can leach/erode from the paint and enter the environment (Karbalaei et al. 2018).
2.2.2 Nonpoint Sources
MP nonpoint sources are more difficult to identify and address. This is because they can enter various areas of the environment through large-scale events such as stormwater runoff, atmospheric deposition from storms, and land application of biosolids (Su et al. 2020). In fact, urban stormwater runoff from heavy precipitation events or floods, winds, and wildfires can lead to MP being removed from various areas all at once, such as construction areas, artificial turf and rubber running tracks, and landfills (Bailey et al. 2021).
The most important sources of MP in the air are determined to originate from synthetic textiles, erosion and abrasion of synthetic rubber tires, and city and household waste (Prata 2018). Other sources of airborne MP include waste incineration, landfilling (Dris et al. 2016), industrial air emissions, sewage biosolids (commonly used in agriculture as a fertilizer), construction materials, and household dryer exhaust (Prata 2018).
Werbowski et al. (2021) studied 12 watersheds around San Francisco Bay. Their results showed MP in all stormwater runoff sampled, with concentrations ranging from 1.1 to 24.6 particles/L (higher than those typically found in wastewater treatment plant effluent). Werbowski et al. (2021) reported fibers and black rubbery fragments (potentially tire and road wear particles) as the most frequently occurring morphologies, comprising approximately 85% of MP in the samples. Liu, Tang, et al. (2019) found MP (primarily P, PE, PP, PS, and PVC) in the water column of stormwater treatment ponds in North Jutland, Denmark. The results showed that ponds capturing highway and residential area runoff had the lowest MP concentration, while ponds serving areas with industry and commerce had the highest. The study also noted that ponds collecting residential stormwater generally held the largest sized MP particles. According to Liu, Tang, et al. (2019), the data demonstrate that stormwater retention ponds act not only as MP sinks but also play a role in the transport of MP from land to the aquatic environment.
2.2.2.1 Tire Wear Particles
Tire wear particles (TWP) have been detected in many environmental media, including ambient air, road dust, and terrestrial soil and sediment, as well as in marine and freshwater aquatic environments (Baensch-Baltruschat et al. 2020, Leads and Weinstein 2019, Panko et al. 2013, Panko et al. 2019, Unice, Kreider, and Panko 2013, Wagner et al. 2018). Some of the chemicals contained in TWP have been reported to have significant impacts on migrating aquatic life (for example, coho salmon, Appendix A.5).
Physical properties, including size, shape, and particle density, will impact the fate and transport of TWP and therefore, overall distribution in the environment (Unice et al. 2019). Fate and transport potential also relies on the regional weather conditions and the soil and hydrologic characteristics of the environment (Wagner et al. 2018). Furthermore, the generation of TWP depends upon the vehicle type, tire type, service life of the tire, and vehicle operations and patterns such as speed and acceleration (Baensch-Baltruschat et al. 2020, Kreider et al. 2010, Wagner et al. 2018). Emitted TWP have the potential to be dispersed in ambient air, then deposit as roadside dust or in roadside soil and be transported to surface water through runoff and erosion then to open water bodies or wastewater treatment facilities (Unice et al. 2019). TWP are generally found more frequently and in higher quantities near roadways and urban areas, particularly those with high-volume traffic patterns (Unice, Kreider, and Panko 2013).
2.2.2.2 Agricultural Sources
Another nonpoint source of MP to terrestrial and marine environments is fragmentation of plastics that are used in agriculture (Karbalaei et al. 2018). MP in agricultural soils have recently gained significant attention in science and society. In addition to sewage sludge and compost, MP are contained in agricultural products (for example, plastic-coated agrochemicals, plastic mulches, silage, and fumigation films, fertilizer sacks) that are added to soils (Karbalaei et al. 2018, Muise 2016). However, knowledge about how much MP has been applied to agricultural soils is scarce (Brandes, Henseler, and Kreins 2021). Modeling efforts indicate that MP distribution in soils is place-dependent, and a spatial understanding of the source materials is needed.
2.3 MP in the Fluvial Environment
Inland waters, urban lakes, and riverbanks have been found to be more susceptible to plastic (macro and micro) pollution, while in the marine environment, ocean current convergence zones, beaches, and seafloors are likely depositional environments for MP. Generally, lakes and inland areas of decreased flow velocity act as MP sinks, whereas rivers and streams serve as MP transport systems. Because MP are translocated by water currents and their vertical distribution is regulated by particle density and biofouling, it is difficult to precisely identify the source of plastic particles in the aquatic environment (Darabi et al. 2021). Aquatic sediments, particularly deep marine sediments, are considered as the final sink of MP pollution. The abundance of MP in deep marine sediment is usually higher compared to terrestrial soil and water bodies (Darabi et al. 2021).
Although plastics in most scenarios have been transported from land to water, they can also be transported from water to land (Moreira et al. 2016, Turrell 2018, Zhang, Chen, et al. 2019, Zhang, Hamidian, et al. 2021, Zhang et al. 2016). Studies suggest that ocean-atmosphere exchange plays a role for the MP in soils (Allen et al. 2020). MP may have been transported to the sea by wind, while it is possible that wave action and wind on the shoreline entrained and carried MP from the shoreline to land. MP may seep into the sea floor and accumulate in the sediment on the bottom of ocean (Barrett et al. 2020). MP may also concentrate in between vegetation—leaves, grasses, and roots in mangroves, seagrass, forests, etc. (Huang, Xiao, et al. 2021).
It is important to note that the transport processes may not be one-way among different media. Figure 2-3 below provides a conceptual transport mechanism among terrestrial land, fresh water, and the marine environment (Horton et al. 2017).
Figure 2-3. Movement of MP in surface waters.
Source: Jonathan McDonald and Todd Miller
2.3.1 Surface Water
MP have been found in freshwater systems across the globe. MP in freshwater systems can originate from a variety of point and nonpoint sources, such as atmospheric deposition, groundwater infiltration, stormwater runoff, and industrial and municipal wastewater discharges. MP concentration in freshwater bodies can vary by orders of magnitude, with urban streams and glaciers having the greatest concentrations of MP (Koutnik et al. 2021). Treatment of industrial and municipal wastewater sources directly affects the volume of MP entering surface water bodies (Appendix A.2, A.4, and A.5).
2.3.1.1 Rivers, Lakes, and Streams
In surface water bodies, MP are prevalent at the surface, throughout the water column, and in sediments, with their distribution dependent on multiple factors, including wind, currents, streamflow rate, and temperature (Petersen and Hubbart 2021). Flow rates in freshwater systems can impact the spatial distribution and concentrations of MP. Areas of decreased velocity can result in accumulation of particles as well as deposition into underlying sediments, whereas areas of high flow rate can result in more transport and possible resuspension of settled particles. Similar to other pollutants, lakes and areas of decreased flow velocity act as sinks, whereas rivers and streams (higher flow velocities) will tend to act as conveyance systems (Petersen and Hubbart 2021). Lakes and low-flow streams in urban areas are particularly susceptible sinks for secondary MP. Rivers transport MP from their sources to the terrestrial boundary and across the boundary to the ocean.
Rivers collect MP from wastewater treatment plant discharges, urban stormwater discharge points, runoff from land surface, and air deposition. Koutnik et al. (2021) found that fiber percentage in the MP samples also varied between locations. The urban water bodies had more fibers than the coastal region, indicating fiber fraction in water decreases downstream. Urban water bodies are expected to contain more fibers because of their release from textile products. Due to their long aspect ratio, fibers can be preferentially removed by aggregation during transport through the environment. Koutnik et al. (2021) also reported that the fiber fraction (compared to plastic particles) increases in water, indicating that fibers are more likely to float in water than nonfibrous MP. Thus, it appears that the shape of the MP, which influences buoyancy, will affect their fate in surface waters.
2.3.1.2 Stormwater
In most urban environments in the western United States, rainfall and runoff wash silt, sediment, and debris into stormwater collection systems that discharge directly to receiving waters without treatment (Moran et al. 2021). Separate stormwater collection systems increase the contaminant load, including MP, that discharge, into our lakes, streams, and bays. MP can be deposited onto the land surface through incidental action (for example, littering, aerial deposition), intentional application (for example, biosolids, agricultural amendments, and plastic covers), and product degradation (for example, tire wear particles). Once present, these plastics and MP can be entrained into stormwater runoff, which then drains to collection systems, lakes, streams, or other low-lying areas. Koutnik et al. (2022) conducted laboratory experiments showing the possibility for freeze-thaw cycles to drive denser MP, such as PET and PVC, to move deeper into the subsurface beneath stormwater treatment systems and consequently elevate groundwater pollution risk.
2.3.1.3 Bays and Estuaries
Bays and estuaries provide an important insight into the fate and transport of MP. Because they act as transitional areas between aquatic and terrestrial habitats and are uniquely located in between fresh and saltwater sources, they are important hubs for MP transportation around the world (Liu, Tang, et al. 2019). Additionally, due to their alternating hydrodynamic conditions, they are considered an important site for understanding the transition of macroplastics into microplastics. This is predominantly due to their ability to act as a sink for larger plastic debris (Yao et al. 2019). Furthermore, MP in wetland environments have been found to be directly derived from the population centers surrounding them (Govender et al. 2020). This means that estuaries contain both primary and secondary sources of MP that can be studied.
Mangrove swamps, productive habitats that act as valuable nurseries for fish and invertebrates, perform as important coastal protection systems and provide crucial natural filtration. These factors make mangroves a vitally important coastal resource for socioeconomic purposes, with thousands of people depending on them for survival (Samidurai, Saravanakumar, and Kathiresan 2012).
Mangroves have dense and developed roots that grow in clay-rich environments that contain a high amount of organic matter. This makes them an important filtration mechanism, ultimately allowing these estuaries to act as MP sinks (Maghsodian et al. 2021, Martin et al. 2020). In fact, the abundance of MP in the mangrove ecosystems has been found to be 4–10 times higher in the water column, and 2–3 times higher in the sediments, than that of other ecosystems (Liu et al. 2022). Studies have also shown that the accumulation of MP in these areas has the ability to alter their environments by negatively affecting various soil properties (Qi et al. 2020).
With mangroves and other estuary ecosystems acting as a large food source and as MP sinks, it may be possible for them to bioaccumulate in the foods that we eat. Crustaceans, mussels, and other deposit feeders living in these environments have an increased chance of ingesting the MP found there (John et al. 2022). Cluzard et al. (2015) studied the key shellfish-growing regions in Baynes Sound, British Columbia, and detected MP in sediments and shellfish tissues. Results showed that MP alter the cycling of key nutrients, such as ammonium within intertidal sediments, and increase the amount of ammonium within the water column. Increased ammonium amounts in the water column can lead to eutrophication and trigger red tides (that is, algal blooms that grow rapidly) that are toxic to marine life and humans due to the chemicals they produce (Cluzard et al. 2015)
2.3.2 Wastewater
Industrial and domestic products have become two of the most rapidly growing sources of MP entering municipal wastewater systems today. Examples of industrial and domestic sources include polymeric fibers released by washing of synthetic clothing (during manufacturing and domestically), manufacturing and use of agricultural products and paints, and manufacturing and use of hand, body, and facial cleansers (Tagg et al. 2015, Zhu, Huang, et al. 2021). Other factors, such as plastic abrasion during dishwashing and MP entering sewage systems during rainfall events, also contribute to the presence of MP in industrial wastewater. This increased use of domestic products containing MP particles increases the chances for MP to reach the environment through direct discharge to water bodies, or as a wastewater treatment byproduct, such as biosolids.
WWTPs play a pivotal role in removal of MP particles before the waste streams are discharged into aquatic environments, yet between 50% and 80% of the global wastewater discharged to aquatic systems remains untreated (Uddin, Fowler, and Behbehani 2020, United Nations 2017). Even when wastewater is treated, there are no standardized treatment or analysis process specifically for MP removal.
The efficiency of MP removal in wastewater treatment plants ranges from 50% to 78% during primary treatment, and between 88 and 99.9% during secondary and tertiary treatment (Flinders 2020), indicating a high potential for capturing most of the MP currently being discharged into aquatic systems today. However, MP removed from wastewater are concentrated in the biosolids produced as a byproduct of the treatment process (Uddin, Fowler, and Behbehani 2020), and can be re-released into the environment through recycling efforts (for example, land application of biosolids, composting operations). Common MP shapes in wastewater, and hence in the biosolids, include fibers from textiles, pellets and flakes from industrial and domestic sources, and films. The land application of biosolids and disposal in landfills are of an increasing concern.
A study published in 2021 detailed that MP taken from wastewater effluents were predominantly (roughly 90%) smaller than 0.5 mm (Ragoobur, Huerta-Lwanga, and Somaroo 2021). The study took place at three WWTP in Mauritius, an island in the Indian Ocean. After water is treated in a Mauritius WWTP, it is discharged to the ocean, surface water, groundwater, or land (with more than 10% being used for agricultural activities). After treated wastewater is used for agriculture, MP has the potential to enter the food chain, and ultimately the human body. As treated wastewater is discharged into the ocean, the MP can circumvent the world’s waterways through the various ocean currents. As a response to the increase in MP in the WWTPs, Mauritius banned the use of plastic bags in the Environment Protection Regulation of 2015, which took effect in March 2021.
Another study published in 2021 details the prevalence of MP entering the South Saskatchewan River in Canada. Most of the MP extracted from the WWTP effluent were found to be synthetic microparticles (for example, MP associated with discharges from routine laundry activities) and were captured using fine-meshed plankton nets (Prajapati et al. 2021). The results showed on average a total of 141 million MP particles being discharged into the South Saskatchewan River every day. The WWTP responsible for the discharge is a Class 4 plant, which is the highest certification in Canada (Prajapati et al. 2021). This study demonstrates that WWTPs may not be effective at removing all of the MP from the treated wastewater, and the authors suggest that there is growing consensus that these particles are going undetected in WWTP effluent (Prajapati et al. 2021).
2.3.3 Groundwater
Based on the limited number of studies, and the fact that many wells are constructed or sampled with a variety of plastic materials, it is difficult to have a full understanding of the prevalence of MP in groundwater (for example, sources, abundances, polymer types, factors). According to Koelmans et al. (2019), groundwater appears to have the lowest abundance of MP compared to other water types (for example, wastewater effluent, lakes, streams, tap water, and bottled water), but more quality data are needed for better comparison due to lack of standard sampling and analysis methods (Koelmans et al. 2019). The environmental factors that impact MP migration in groundwater include particle size, density, soil moisture content, pH, salinity, and ionic strength.
MP in groundwater have been documented in limited studies, which have reported the presence of MP in groundwater systems in the state of Illinois and in Germany and South Africa (Bouwman et al. 2018, Mintenig et al. 2019, Panno et al. 2019). In these studies, the reported average concentrations ranged from 0.0007 particles/L (in Germany) to 15.2 particles/L (fibers reported in Illinois). The size of MP ranged from 0.45 µm (Illinois) to greater than 1.5 mm (Potchefstroom, South Africa) for fibers, and 50 µm (Germany) to 600 µm (Potchefstroom, South Africa) for fragments. MP have been found in shallow and deep groundwater. Mintenig et al. (2019) identified MP such as P, PA, PE, PVC, and epoxy resin (in sizes of 50–150 µm) in raw water extracted from wells approximately 30 meter deep near Hodorf, Germany. As mentioned above, due to lack of standard sampling and analysis methods, the quantity, types, and sources of MP reported under these types of scenarios should be further verified.
The Shi et al. (2022) study in northern China indicates that MP, including PA, PE, PP, PS, and PVC, were found in all of the groundwater samples collected, with the higher concentrations reported around sewage treatment plants, landfill sites, and vegetable production sites. Microplastics ranged in size from less than 20 μm to greater than 500 μm, and the largest was 2,500 μm. Eighty percent of MP particles sizes were less than 50 μm, consisted of fragments and fibers, and had a transparent color. The study found a correlation between MP and antibiotics (antibiotics in groundwater can result from WWTP discharges) and concluded that the MP can bind to antibiotics “through hydrogen bonding, hydrophobic interaction, van der Waals forces and electrostatic interaction mechanisms,” and can reduce the decomposition of antibiotics in soil and water.
Panno et al. (2019) concluded that crevices, conduits, branch-work caves, fractures, and sinkholes within geological formations may act as preferential pathways for MP migration from surface water to groundwater. Due to the irregular surfaces of fractures and crevices from retardation and the filtration effect, only the smaller size fractions of MP would migrate into groundwater. Bouwman et al. (2018) in their studies concluded that the size class profiles seem similar between soils and groundwater (size decreasing over the depth of soil or groundwater where MP are present); however, in groundwater, fragments found were smaller, whereas fibers found varied broadly in their sizes. The results suggest that groundwater susceptible to pollution by surface water may be more likely to contain MP.
Re (2019) indicated that MP can be introduced into an aquifer from losing streams, where stream water recharges the aquifer, and can be introduced into a deeper aquifer by managed aquifer recharge or aquifer storage and recovery systems, such as injecting treated wastewater containing MP, or surface runoff or stream water containing MP (Re 2019). Li, Wang, et al. (2021) investigated the impacts of seawater intrusion and groundwater-seawater displacement on the transport behaviors of marine plastics and concluded that the seawater intrusion would transfer MP into coastal aquifers. The available studies seem to be able to quantify only the MP entering groundwater, while the fate of MP in groundwater remains limited (Boyle and Örmeci 2020, Gao et al. 2021).
Figure 2-4 provides a schematic representation of possible pathways for MP to enter and be transported through an aquifer.
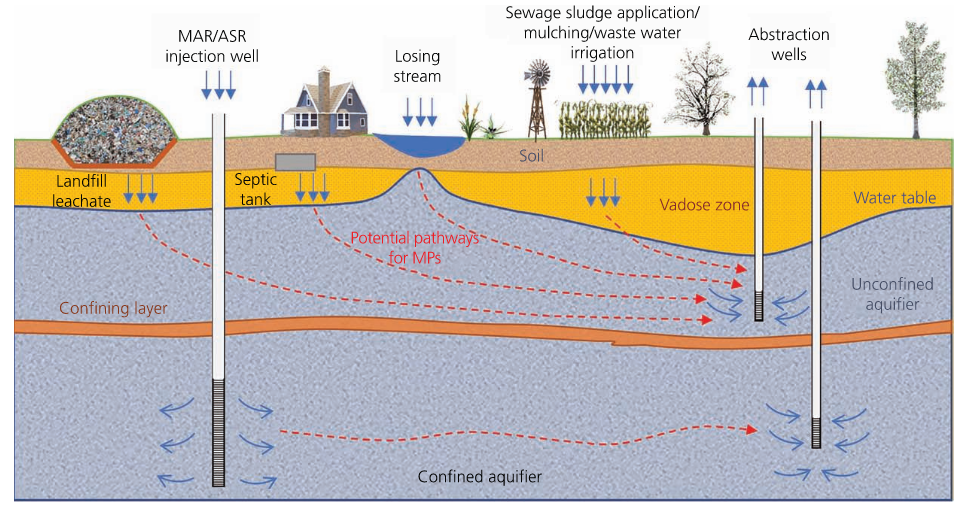
Figure 2-4. Schematic representation of MP transport pathways in an aquifer.
MAR (managed aquifer recharge), ASR (aquifer storage and recovery)
Source: O’Kelly et al. (2021).
Smaller particles preferentially migrate deeper in soil and groundwater. The wet-dry cycle and infiltration play an important role in the depth to which MP penetrate the subsurface (Gao et al. 2021, O’Connor et al. 2019). It also should be noted that underground anthropogenic activities may introduce MP in groundwater (for example, various types of well construction and groundwater investigation, etc.) when plastic material, such as PVC casings/screens, low-density polyethylene (LDPE) tubing, etc., is used.
2.3.4 MP in the Oceans
MP are present in oceans around the world. MP enter the oceans from our estuaries, rivers, industrial outfalls, and the atmosphere. On the ocean surface, gyres (the major ocean currents) move MP around the ocean, where they eventually move toward the slower moving centers (Figure 2-5). Depending on their buoyancy, MP may remain at the surface for many years.
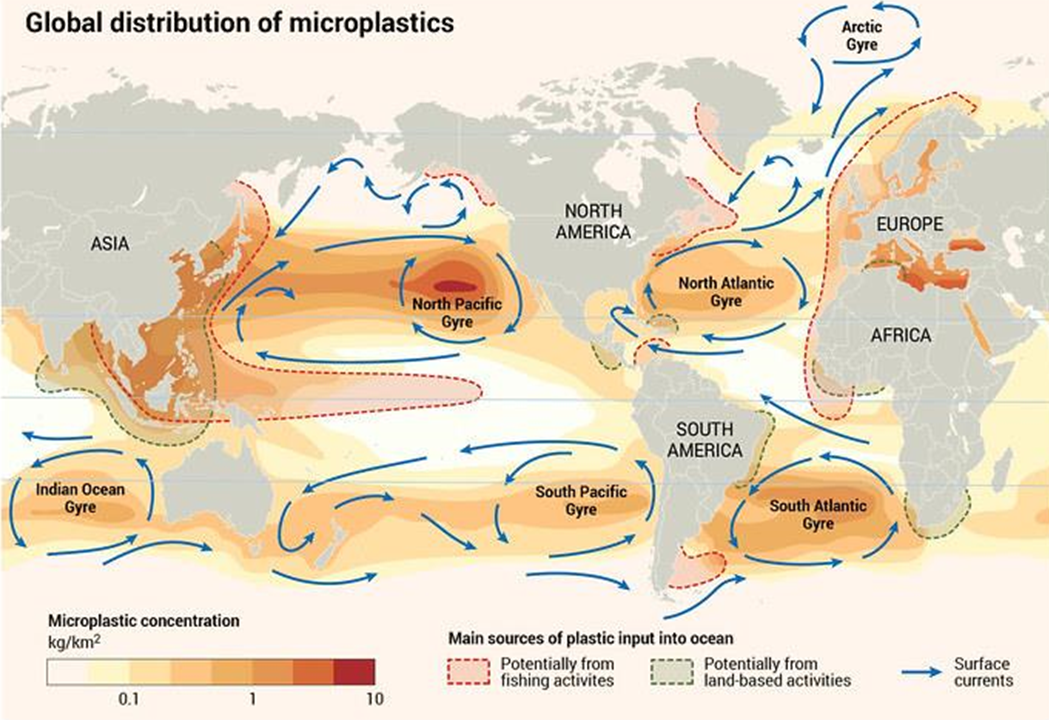
Figure 2-5. Global distribution of microplastics.
Source: Riccardo Pravettoni and Philippe Rekacewicz,
https://www.grida.no/resources/13339
Denser MP sink deep into the ocean and are distributed throughout the subsea by currents. MP have been found at every depth in the ocean where scientists have looked. The Monterey Bay Aquarium Research Institute reported MP throughout the deep pelagic water column with the highest concentrations present at depths between 200 m and 600 m (Choy et al. 2019).
There are many physical processes governing transport of floating plastic debris, such as ocean currents and convergence zones, Stokes drift, tides, wind force, Langmuir circulation, ice formation, melting and drift, etc. (van Sebille et al. 2020). Vertical transport is also possible due to degradation or aggregation with other denser particles (Fazey and Ryan 2016). Plastic particles less dense than seawater are transported throughout marine environments (Zarfl and Matthies 2010) and concentrated in the five great garbage patches in the Indian, Atlantic, and Pacific Oceans (Lebreton et al. 2018). Plastics on the ocean surface will break down through physical and biological processes to produce MP. The resulting MP exhibit positive, neutral, or negative buoyancy depending on the inherent physical properties (for example, density) of the plastic and changes in density due to aggregation or biological fouling (Cole et al. 2016, Kaiser, Kowalski, and Waniek 2017, Long et al. 2015, Van Cauwenberghe et al. 2013).
Transport of MP in deep marine environments is driven by three primary processes (Kane and Clare 2019):
- gravity-driven transport in sediment-laden flows
- biologically driven settling or transport of surface-floating or water column–suspended particles
- transport by thermohaline currents, including settling and reworking of deposited MP
Along the coastline, MP can be ejected into the atmosphere through convective updrafts when breaking waves cause bubbles of trapped air to rise to the surface and burst. The resulting void caused by the bursting bubble is quickly backfilled by the water causing a secondary ejection known as a jet to eject MP into the atmosphere (Allen et al. 2020). According to the 2020 article, an estimated 136,000 tons/year of MP could be ejected into the atmosphere from sea spray.
2.4 MP in Soil
Soil may represent the largest global reservoir for MP, although most MP research has been concentrated on the marine environment (Hurley and Nizzetto 2018). It is likely that soils will act as long-term sinks for MP given that most plastics are used and disposed of on land (Horton et al. 2017). Plastics are suggested as a stratigraphic indicator for the past hundred years due to their suspected omnipresence in soils (Zalasiewicz et al. 2016).
2.4.1 MP Distribution in Soil
MP spiral around the globe with distinct atmospheric, oceanic, cryospheric, and terrestrial residence times, and have been found in soils from urbanized cities to remote and isolated areas such as the Tibetan plateau (Allen et al. 2020, Brahney et al. 2021).
The abundance in soils depends on soil type and management, plastic size and density, and precipitation (Horton et al. 2017, Nizzetto, Futter, and Langaas 2016). The types and categories of MP in soils depend on land use, regions, and other factors. Lands close to busy roads and those used for waste management and agricultural areas, as well as home gardens, are found to exhibit high MP abundance (Horton et al. 2017, Huerta Lwanga et al. 2017). Results of the study in southeast Germany by Piehl et al. (2018) where soils from agricultural land receiving conventional agricultural treatment (no MP-containing fertilizers or agricultural plastics, such as greenhouses, mulch, silage films, biosolids, etc., were used) suggested that PE was the most common polymer type, followed by PS and PP. Films and fragments were the dominate MP categories (Piehl et al. 2018). Another study (Xu, Han, et al. 2022) conducted in China showed slightly different results regarding the shape and type distribution of MP in soil. PE and PP, followed by rayon and PET, comprised the majority of polymers in soil among 25 types detected, and film was the most abundant type compared to fragment, fiber, foam, and pellet. The categories of MP are expected to be different at nonconventional agricultural treatment farmland and the MP concentrations are expected to be higher. For example, the number of fibers may be higher in the soil where biosolids are applied as fertilizer.
The size distribution of MP in soil is affected by the various pathways in which they enter the environment. For example, fibers, mainly in the size range of 0.2 mm to 0.66 mm, are observed in urban and suburban regions during atmospheric deposition (Dris et al. 2016). The distribution of MP in soils also depends on soil depth. The study by Piehl et al. (2018) indicated that the plastics found in the upper 50 mm of the soil are commonly several orders of magnitude larger than MP (that is, greater than 5 mm in size), and the abundance of particles increases with decreasing size. This is likely due to progressive fragmentation of larger pieces into more and smaller pieces.
2.4.2 Sources of MP in Soil
Major sources contributing MP to soils include landfills, litter along roads and trails, illegal waste dumping, road runoff (for example, TWRP), WWTP by-products (for example, reclaimed water irrigation, biosolids applications, see Figure 2-6), agricultural products and processes (for example, mulch, fertilizer, soil conditioners, pesticides, seeds, planting aids, direct use of plastic sheets, etc.), dredged or remediated sediments (Pathan et al. 2020, Wiesmayer 2021), and atmospheric deposition (Brahney et al. 2021). In addition, intentionally added MP are commonly used in both agriculture and horticulture, mainly from the nutrient prills (for example, polymer-coated fertilizers) for controlled-release fertilization (GESAMP 2015).
It was estimated that between 125 and 850 tons of MP per million inhabitants are added annually to European agricultural soils through direct application of biosolids; this includes a total yearly input to farmlands of 63,000–430,000 tons of MP in Europe and 44,000–300,000 tons of MP in North America (Nizzetto, Futter, and Langaas 2016). The number of MP (for example, synthetic fibers) entering the terrestrial environment has been growing and is expected to continue to increase (Gavigan et al. 2020).
Direct use of MP-containing materials is another pathway for MP into soil, such as the application of biosolids generated from a WWTP. For example, as textiles are washed, fibers can be broken and the small pieces (MP) are entrained into the wastewater that is discharged to the local WWTP. The MP (microfibers) are accumulated in the biosolids after the treatment process. The biosolids are then partially dried and then can be applied to agricultural fields as fertilizer (Brahney et al. 2021, Kapp and Miller 2020, Lant et al. 2022). It was noted that synthetic/cotton blends actually produce the largest quantities of MP (such as PE/cotton), when compared to the synthetic material alone (Zambrano et al. 2019). It was estimated that approximately 16%–38% of the heavier-than-water MP contained in the biosolids were added to and stored in soils (for example, through routine applications of sewage biosolids) based on a mathematical model of catchment hydrology, soil erosion, and sediment budgets (Nizzetto et al. 2016). MP have also been intentionally added to agricultural products to control the release of fertilizers or increase the water-retaining capacity of soils. Recent scientific studies show that agricultural soils could hold more MP than the ocean basins, potentially making agricultural lands some of the most plastic-impacted places outside of landfills and urban spaces (Pukclai 2020)

Figure 2-6. Biosolids being applied to an agricultural field.
Source: City of Los Angeles Environment and Sanitation.
Fragmentation of intentionally or unintentionally discarded plastic debris is one of the major input routes (Bläsing and Amelung 2018, de Souza Machado, Kloas, et al. 2018, Horton et al. 2017, Hurley and Nizzetto 2018, Piehl et al. 2018). During this process, partially trapped MP at the soil surface are directly exposed to UV light and broken down through photodegradation, which is recognized as a major process for decomposition of polymer materials (Singh and Sharma 2008, Williams and Simmons 1996). For those already broken down, further mechanical disintegration caused by application of shearing forces can occur through other mechanisms depending on site-specific locations, such as freeze-thaw cycles, pressure due to burial under soil or snow, or damage caused by interactions with organisms (Lambert, Sinclair, and Boxall 2014, Rillig 2012). In farmland, shearing forces can be ploughing and tillage work (Piehl et al. 2018). It is worth noting that the study by Weber, Santowski, and Chifflard (2022) on the agricultural fields after sewage biosolids application concluded that anthropogenic ploughing was mainly responsible for plastic spreading, as opposed to natural transport processes such as erosion, although most MP remained spatially stable over long periods of time and only some were physically transported.
2.4.3. Fate and Transport of MP in Soil
The fate and transport mechanisms of MP to and through soil are not well understood (Dris et al. 2016). Available studies suggest that the fate of MP in soils is complex, and many factors play a role, such as the type of MP (that is, polymers vs. others), density (affecting the wind action transport pathway and movement potential), size, color, shape, soil conditions (for example, pH, mineral content, organic matter, etc.), weather conditions (that is, greater wet-dry cycles increase the migration depth), etc. (Boyle and Örmeci 2020, Horton et al. 2017, Huerta Lwanga et al. 2017, O’Connor et al. 2019). Several pathways were documented for larger plastic debris entering terrestrial soils and later becoming MP (Bläsing and Amelung 2018, Brahney et al. 2021, Horton et al. 2017, Huerta Lwanga et al. 2017, Hurley and Nizzetto 2018, Piehl et al. 2018, Rillig, Ziersch, and Hempel 2017).
Wind erosion and transport constitutes another distribution route for small plastics and MP from their original source to remote areas where deposition to soils (due to rain, declining wind, or barriers) could occur (Dehghani, Moore, and Akhbarizadeh 2017, Dris et al. 2017). Particle motion during the erosion and transport process includes pushing or rolling over the surface, also known as “surface creep” (Piehl et al. 2018).
Previous studies also demonstrated that MP could be transported along the soil profile by earthworms and via preferential flow paths (Huerta Lwanga et al. 2017, Rillig, Ziersch, and Hempel 2017). The lower soil could be hydraulically connected to the upper soil, particularly in clayey soils that tend to shrink while drying (Bogner et al. 2013). MP can travel downward, reaching groundwater, especially when the groundwater table is shallow. Once they encounter the saturated soils, the MP can flow through preferential pathways to other receptors (Piehl et al. 2018).
2.4.4 Impact of MP on Soil Matrix
Studies have documented that MP induce impacts on soil matrices, depending on their shape (for example, particles, films, fibers), concentrations, or properties (that is, chemical composition, dimensions). MP may alter biological, chemical, and physical properties of soils (Pathan et al. 2020). The interactions between MP in soils and soil microbiota, fauna, and vegetation may further disrupt the biophysical environment of farmland soil, potentially leading to economic losses and to their entrance into the trophic food change, affecting human feeding and health (Pérez-Reverón et al. 2022).
Once MP enter soils, the abundance, distribution, and physio-chemical properties of MP may change via various mechanisms. MP may cycle through a new media or cycle back to the source media. MP may cycle from soils back to surface water bodies through erosion and stormwater transport, or back to the atmosphere then to surface water bodies or the ocean through aerial deposition, or migrate vertically into groundwater, or further degrade. MP have the potential to alter soil properties and processes, such as bulk density and water retention capacity (de Souza Machado, Lau, et al. 2018). MP films accelerate evaporation, decrease water content, and reduce the soil tensile strength and bulk density while increasing soil porosity.
Lehmann et al. (2021) examined the effects of introduced MP of varous shapes and composition on soil structure. MP fibers have a distinct negative impact on soil dissimilarity compared to foams, films, or particles. Polyester microfibers and foams can cause negative effects on soil aggregate stability, new aggregate formation, and concentrations of larger aggregates, while MP beads and particles may cause a range of positive to negative effects (Boots, Russell, and Green 2019, de Souza Machado, Kloas, et al. 2018, de Souza Machado et al. 2019, Lehmann et al. 2021, Liang et al. 2019, Zhang, Chen, et al. 2019). Microfibers can introduce fracture points after becoming a part of newly formed aggregates, likely facilitating breakdown when encountering external force. The effect is also related to the concentration and dimensions of microfibers (de Souza Machado, Kloas, et al. 2018, Lehmann et al. 2021). Particles are less well incorporated into aggregates compared to fibers, likely due to size difference and other properties, such as surface roughness, irregularity, rigidity, and brittleness (Lehmann et al. 2021). The 2021 study by Lehmann et al. also indicated that MP films negatively affect aggregate formation, but positively affect aggregate stability. The size of films likely plays a role in the stability effect (Lehmann et al. 2021).
MP may become a part of soil aggregates after entering the pore networks and may control soil processes, such as biogeochemical cycles, soil carbon storage, and processing (Rabot et al. 2018). Also, diverse types of MP (with different chemical properties), due to their potential toxicity (that is, additives leaching and migration), can affect soil microbial activity, subsequently affecting soil aggregation because microbial metabolites can function as a gluing substance and promote soil stability (Caesar-Tonthat 2002).
The addition of plastic granules can increase the total organic carbon content of soil (Rillig 2018). Also, MP films and particles can decrease the decomposition of organic matter in soil; this effect is more detectable for casted PP, PE films, and PP particles compared to other types (PET films, PC particles, etc.) and shapes (foams and fibers) studied (Lehmann et al. 2021). This effect is likely due to the change of soil physical parameters (that is, porosity, connectivity, aeration) and sorption and migration of chemicals and additives, resulting in microbial activity changes, and subsequently changes the mineralization rate of soil organic matter (Lehmann et al. 2021, Zhang, Zhang, and Li 2019). Addition of MP in soil can negatively affect the nutrient availability by increasing the rate of dissolved organic matter decomposition in soil, therefore decreasing the dissolved organic carbon, nitrogen, and phosphorus in soils (Liu et al. 2017).
2.5 MP in Sediment
MP have been detected in marine and freshwater sediments and in both flowing and nonflowing systems. The primary source of MP in sediment is the settling out of suspended MP in the water column. MP characteristics (density, diameter, and shape), wind, tides, biofouling, and weathering influence particle settling (Darabi et al. 2021). Fibers and fragments are frequently detected MP in sediments, and higher density MP are more likely to be trapped and settle in sediment (Darabi et al. 2021, Falahudin et al. 2020). MP are more likely to settle in surficial sediments, and MP abundance decreases with sediment depth. Recent modeling indicates that residence times are highest in river headwaters and can be years under low-flow conditions (Drummond et al. 2022). MP in river sediments are often unaccounted for and are likely a pollution legacy that is crucial to include in future global assessments (Drummond et al. 2022).
A study by Falahudin et al. (2020) found that MP were more concentrated in clay as opposed to sand-dominated sediments. This study also assessed the importance of total organic carbon (TOC) impact on the transportation of MP and suggested that sediments with higher TOC content are more likely to have a higher abundance of MP. Maes et al. (2017) found a correlation between abundance of MP with decreased sediment particle size. However, a study by Vermaire et al. (2017) found that the concentration of MP recovered in sediment samples was not significantly related to sediment particle size or TOC content.
MP in sediment can be resuspended and transported through surface water flow to downgradient areas. Thus, rivers are considered a key pathway for transporting MP to different areas (Darabi et al. 2021), particularly to coastal areas (Kane and Clare 2019). In ocean systems, MP can be transported and deposited by turbidity currents (Darabi et al. 2021, Pohl et al. 2020). MP entrained in the flow are transported along the bed, or in suspension (Darabi et al. 2021), and deposited (redistributed) downstream. The depositional process is in part governed by size and density of the plastic (Sutton et al. 2016).
2.6 MP in Air
The atmosphere plays an important role in MP transport, with increased occurrence and higher transport concentrations noted in more densely populated areas (Petersen and Hubbart 2021, Zhang, Kang, et al. 2020). This increase is attributable to greater anthropogenic activity, industrialization, and human population density. Atmospheric deposition of MP may be driven by precipitation events, including both rain and snow (Allen et al. 2019, Dris et al. 2016). Currently, due to their inhalation and combination with other pollutants (for example, mercury, polycyclic aromatic hydrocarbons), MP are considered an emergent component of air pollution (Barboza et al. 2018, Gasperi et al. 2018, Liu, Li, et al. 2019, Rochman et al. 2019, Tourinho et al. 2019, Wright and Kelly 2017).
Airborne and atmospheric transport of MP was first reported in Paris during 2015 (Dris et al. 2015). Atmospheric transport includes numerous processes (for example, wind speed, up/down drafts, convection lift, and turbulence) that are all considered important vectors affecting MP transport. The vectors further influence the flux mechanism and source-sink dynamics of plastic pollution in both marine and terrestrial environments (Bank and Hansson 2019, Liu, Wang, et al. 2019, Zhang, Gao, et al. 2019). While investigating MP contamination in urban settings, Dris et al. (2015) concluded that atmospheric fallout could be a significant source of fibers in freshwater ecosystems. MP have also been detected in the atmosphere in pristine remote areas far away from source regions, suggesting potential long-distance atmospheric transportation. Recent studies reaffirm that atmospheric MP transport constitutes a major pathway for MP to remote regions (Bank and Hansson 2019, Evangeliou et al. 2020). The Brahney et al. (2021) atmospheric transport model revealed that MP deposition to the terrestrial environment over the western United States came approximately 84% from roads, 11% from sea spray, and 5% from agricultural soil dust. In addition, approximately 0.4% of MP carried by dust in urban areas resulted from road traffic (for example, TWP) and litter breaking apart (Brahney et al. 2021).
Various shapes, including fiber, fragment, and film, have been detected in the atmosphere (Abbasi et al. 2019, Allen et al. 2019, Bergmann et al. 2019, Dehghani, Moore, and Akhbarizadeh 2017, Klein and Fischer 2019). Fibers and fragments appear to be the dominant shapes. Fibers, for example, are the dominant shape found in urban atmospheric deposition and are likely closely connected to the increasing production of synthetic fibers for clothing, upholstery, carpet, etc., while fragmented MP could possibly result from the exposure of larger plastic items to strain, fatigue, or UV degradation (Liu, Wang, et al. 2019). However, identifying the difference between fibers and fragments for smaller MP may be difficult due to their size (Zhang, Kang, et al. 2020). MP have also been detected in indoor air due to sources like building materials and consumer goods, with fiber being the predominant shape of MP in most indoor dust samples (Zhu et al. 2022).
The predominant size of atmospheric MP is near the smaller end of the scale, where fibers and fragments have been investigated. In pristine, remote locations, the predominant length of plastic fibers was less than 300 µm with a greater proportion of fragments sized less than 50 µm. In urban locations, fiber lengths were predominantly between 200 µm and 5,000 µm, whereas most fragments were less than 63 µm (in the longest direction). The amount of MP particles decreases with increasing size; particle size is an important aspect of atmospheric analysis and research because it affects their deposition (Bergmann et al. 2019, Isobe et al. 2015).
To date, there is no clear correlation or explanation for the variability or composition of polymer types in atmospheric samples. The variety of polymer types found in atmospheric samples published to date does not indicate a clear or obvious delineation between lighter and denser polymer types (Zhang, Kang, et al. 2020)
2.7 MP in Urban Litter
Studies as early as 2008 (DOEE 2008) have shown that a significant amount of urban litter is plastics. San Francisco Bay Area monitoring studies conducted on behalf of the Bay Area Stormwater Management Agencies Association (BASMAA) have characterized urban litter, including macroplastic items that will break down into MP in the environment. Over 150 storm drain trash capture devices were monitored between 2010 and 2011 (EOA 2014). Trash and debris were intercepted, collected, and characterized three to four times at each inlet. Overall, plastic items made up between 2.2% and 15.1% by volume (0.3%–3.0% by weight) of the material captured during the four storm events. Miscellaneous trash, which includes cigarette butts (a source of MP) and items made of rubber, fabric, or other hybrid materials, comprised 0.1%–4.8% by volume (0.9%–1.5% by weight) during the same four events. These values can change dramatically based on geography. A 2021 study (Youngblood, Finder, and Jambeck 2021) reported that 74% of the litter, by count, in the Mississippi River basin is plastic.
Storm events likely play a major role in mobilizing macroplastics and MP derived from litter. A Southern California study evaluating inputs from the Los Angeles River drainage to the coastal ocean near Long Beach found that concentrations of MP increased sevenfold following a storm, from 8 pieces/m3 to 56 pieces/m3 (Moore, Lattin, and Zellers 2005). Discharge of MP to San Francisco Bay via the Sacramento-San Joaquin River delta has not yet been evaluated; however, studies of its tributaries and watersheds indicate that stormwater plays a major role in transport of MP to the bay (Sutton et al. 2019). Studies of tributaries to Chesapeake Bay and the Great Lakes suggest that they can be a significant pathway for MP pollution (Baldwin, Corsi, and Mason 2016, Moore, Lattin, and Zellers 2005, Yonkos et al. 2014). Surface waters of four tributaries to Chesapeake Bay (Appendix A.2) were monitored for MP monthly between July and December to assess relative loads and the influence of storms on the loads (Yonkos et al. 2014). All but one of the samples collected contained MP, ranging in concentration from less than 1 to greater than 560 g/km2. The highest concentrations were associated with heavily urbanized areas after storm events (Yonkos et al. 2014). A study of 29 Great Lakes tributaries, each sampled three or four times, found that 98% of plastic particles were MP (Baldwin, Corsi, and Mason 2016). Fragments, films, foams, and pellets were found at higher levels in tributaries draining urban watersheds, and during conditions leading to runoff, such as rainfall or snowmelt. Interestingly, fibers, the most frequently detected particle type, were not associated with urban areas, wastewater discharges, or runoff (Baldwin, Corsi, and Mason 2016)
2.8 Prevalence of MP in Biota
MP have been found in terrestrial and marine biota, including plants, invertebrates, birds, mammals, and fish, spanning all levels of the food web. It is important to note that there are still conflicting views on the ability of MP to bioaccumulate and biomagnify in organisms. For example, some studies have found that bioaccumulation is clearly taking place among trophic levels (Miller, Hamann, and Kroon 2020), while others suggest that although it is clear that MP are being ingested, there is no clear data proving they are bioaccumulating or biomagnifying (Gouin 2020). Additional discussion of trophic transfer can be found in Section 4.4.
Studies have shown that plants do uptake MP (Petersen and Hubbart 2021). In a study of crop plants, MP were found to be taken up by roots and then transported to the shoots (Li, Luo, et al. 2020). MP can also be transported to the fruit and have been detected in tomatoes (Hernández-Arenas et al. 2021). Earthworms can transport and disperse MP in soils through the external attachment of the plastic particles to the animals or by ingestion and subsequent egestion (Allen et al. 2022, Petersen and Hubbart 2021). However, transport and movement rates of soil MP are poorly documented and factors influencing these rates remain largely unknown (Allen et al. 2022, Petersen and Hubbart 2021). MP can also be consumed by soil invertebrates, and MP can accumulate in the digestive tract of soil organisms (Petersen and Hubbart 2021).
Cera and Scalici (2021) recently reviewed the available literature evaluating MP in freshwater biota. A total of 62 publications were identified evaluating MP in biota worldwide. Figure 2-7 illustrates the frequency of studies according to taxa (more information about biota sampling can be found in Sections 3.4.4 and 3.6.4.
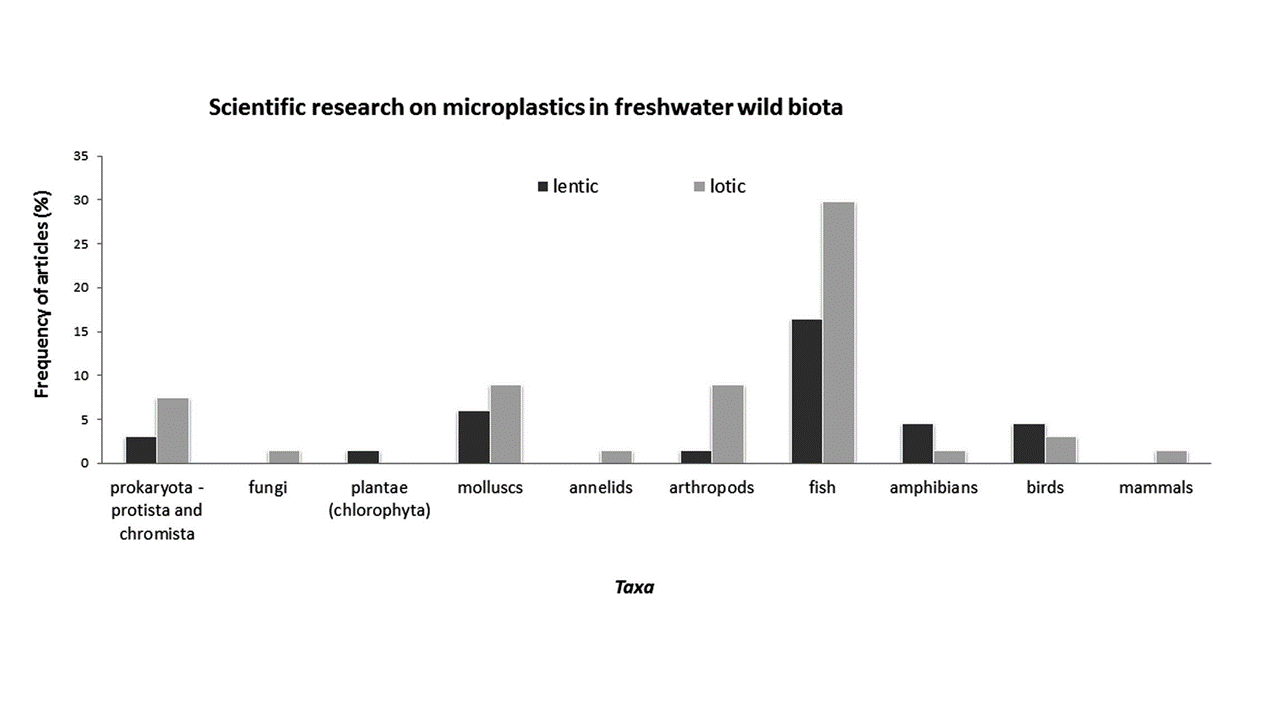
Figure 2-7. Number of studies on microplastics in freshwater biota.
Source: Cera and Scalici (2021).
Among the different feeding types susceptible to ingesting MP, suspension-feeding organisms that typically feed on particulate matter suspended in the water column and filter-feeding organisms that filter large volumes of water may be at greater risk to MP exposure, although some suspension-feeding fish can recognize MP as inedible material (Flinders 2020). Ingestion of MP has been documented in a wide range of freshwater organisms, including protozoans, rotifers, planktonic crustaceans (de Sá et al. 2018, Flinders 2020, Scherer et al. 2020), and insect larva (Al-Jaibachi, Cuthbert, and Callaghan 2019).
MP are consumed by marine and freshwater fish (Bellas et al. 2016, Choy and Drazen 2013, Leslie et al. 2017) and benthic macroinvertebrates (Windsor et al. 2019), and terrestrial organisms. Once ingested, organisms facilitate transport of the MP in the environment (Rillig, Ziersch, and Hempel 2017). MP were found in species spanning all levels of marine food webs in the Monterey Bay pelagic ecosystem, at depths ranging from 5 m to more than 1,000 m (Choy et al. 2019). There are insufficient data collected to date to develop a comprehensive understanding of the full distribution or fate of MP in ocean waters. However, Choy et al. (2019) concluded that their findings suggest that one of the largest and currently underappreciated reservoirs of marine MP may be contained within the deep sea.
2.9 Degradation
Plastics are subjected to abiotic and biotic degradation processes involving chemical, physical, and biological reactions in the environment, which result in the formation of secondary MP. Many advanced technologies have been developed to characterize the degradation of plastics. Light induces the photodegradation of polymers and is additionally linked to the chemical reactions that take place due to the carbonyl groups absorbing the light’s UV radiation. Degradation causes oxidation and chain scission of plastic polymers, forming low molecular weight degradation products and ultimately causing changes in physicochemical and mechanical properties of the MP (Gewert, Plassmann, and MacLeod 2015). Photoaging can have significant effects on the properties of MP, leading to changes in their interaction with the surrounding environment. By changing their size, structure, and density, there is a wider distribution net, allowing MP to spread to every corner of the earth (Cheng et al. 2022)
2.9.1 Abiotic Degradation
Thermal degradation refers to the breakdown of plastics due to energy input stemming from elevated temperature. Plastics can undergo thermo-oxidative reactions at high temperature. When sufficient heat is absorbed by the polymer to overcome the energy barrier, the long polymer chains can be broken, generating radicals (Petersen and Hubbart 2021, Pirsaheb, Hossini, and Makhdoumi 2020). These radicals can react with plastic, resulting in further breakdown or chemical change.
Mechanical degradation refers to the breakdown of plastics due to the action of external forces. In the environment, external forces can come from the collision and abrasion of plastics with rocks and sands caused by wind and waves. Freezing and thawing of plastics in aquatic environments can also result in the mechanical degradation of polymers (Niaounakis 2015). Farming practices (for example, plowing fields, physically moving/removing plastic barriers) can generate MP from existing products. The effect of the external forces depends on the mechanical properties of the plastics.
In air, one study showed photodegradation of plastics resulted in changes in appearance and texture, a decrease in mechanical properties, and alteration of physicochemical properties (Ojeda et al. 2011). Plastics in air sheltered from UV also showed color changes during exposure, and visible light and nitrogen dioxide (NO2) were found to be the most important variables related to the degradation, followed by ozone.
2.9.2 Biotic Degradation
Although many plastics are typically resistant to biodegradation, some organisms (for example, bacteria, mealworms) in soils have the ability to degrade specific polymers (Gu 2003, Yang, Yang, et al. 2015). For example, while photodegradation is mainly responsible for the initial degradation of plastics floating on the surface of seawater, biodegradation may take over once the plastic surface is covered with biofilm (Zhang, Hamidian, et al. 2021). Biological degradation can occur through physical breakdown of the plastics by organisms, such as by biting, chewing, or digestive fragmentation (Cadée 2002, Cau et al. 2020, Dawson et al. 2018, Jang et al. 2018, Mateos-Cárdenas et al. 2020, Porter, Smith, and Lewis 2019) or by biochemical processes (Danso et al. 2019). Microorganisms, including bacteria, fungi, and insects, contribute to the biological degradation of plastics (Crawford and Quinn 2017). Plastics may be very persistent on the seafloor and in marine sediment due to low temperatures and dissolved oxygen concentrations resulting in slow biodegradation processes, as well as protection from ultraviolet radiation.